��Ŀ����
X��һ����Ҫ�Ĺ�ҵԭ�ϣ� X��160��ֽ��Ʊ�������Ͱ�������Ӧ����ʽΪ��X  HCNO + NH3������ƽ�������������գ�
HCNO + NH3������ƽ�������������գ�
��1����������Ӧ���漰�ĸ�Ԫ���У�ԭ�Ӱ뾶����Ԫ����_________����ԭ�Ӻ�����ӹ�ռ��_______�������
��2��������������������Ԫ�ش���ͬ���ڣ�������������˵������Ԫ��C,N,O�ǽ����Եݱ���ɵ���ʵ��____________��
a������������Ӧˮ���������
b��������H2��Ӧ�����׳̶�
c�������γɻ�������Ԫ�صĻ��ϼ�
��ҵ���ڴ��������£���NH3��Ϊ��ԭ���������е�NOx��ԭ�����ĵ���ˮ����Ӧ����ʽ�ɱ�ʾΪ��
2NH3��g����NO��g����NO2��g�� 2N2��g����3H2O��g��
2N2��g����3H2O��g��
��3��һ�������¸÷�Ӧ���ݻ�Ϊ2L�������ڷ�Ӧ��20 minʱ�ﵽƽ�⣬����N2 0.4 mol����ƽ����Ӧ���ʦ�(NO)��__________mol/L��min��
��4�����÷�Ӧ�ﵽƽ��ʱ������˵��һ���������___________��
a�������ܶȲ��� b��������ɫ����
c����(NO)��= 2��(N2)�� d��N2��NH3��Ũ�����
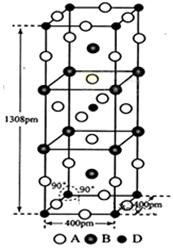
��5��X���Դ���NH3��ԭ�����е�NOx��X�ķ���ģ����ͼ��ʾ��X����ʽΪ______________��
��6�����������ֽṹ��һ�ַ����ں�����������Ϊ���ᣬ��һ�ַ����ڲ�����������Ϊ�����ᣬ�����ֽṹ������ԭ���������Ѵﵽ�ȶ��ṹ��������Ҳ������״�ṹ����ֱ�д�������������Ľṹʽ��_______________��______________��
��1��C��1�֣���4����1�֣�
��2��c��2�֣�
��3��0.005mol/(L��min) ��2�֣�
��4��c��2�֣�
��5��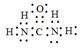 ��2�֣�
��2�֣�
��6��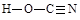
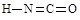 ��2�֣�
��2�֣�
�����������������X  HCNO + NH3������ƽ����Ӧ����֪X�к���C,H,O,NԪ�ء�
HCNO + NH3������ƽ����Ӧ����֪X�к���C,H,O,NԪ�ء�
��1����Ԫ���У�ԭ�Ӱ뾶����Ԫ����̼����ԭ�Ӻ�����ӹ�ռ��4������ֱ���1S22S22P2������S���2����P���2����
��2��Ԫ�طǽ����Եݱ���ɵ���ʵ����ͨ��c�������γɻ�������Ԫ�صĻ��ϼ����жϣ�������Ԫ��û����������Ӧˮ�����Ҳ��ͨ��������H2��Ӧ�����׳̶����жϡ���ȷ��Ϊbc��2NH3��g����NO��g����NO2��g�� 2N2��g����3H2O��g��
2N2��g����3H2O��g��
��3��һ�������¸÷�Ӧ���ݻ�Ϊ2L�������ڷ�Ӧ��20 minʱ�ﵽƽ�⣬����N2 0.4 mol����ƽ����Ӧ���ʦ�(NO)��0.4/(2*20)*2="_0.005mol/(L��min)" ��
��4��a�������ܶ�ʼ�ղ��䣬������Ϊ�ж����ݡ�b��������ɫ���伴Ũ�Ȳ��䣬��ȷ��c����(NO)��= 2��(N2)����ֵ��ϵ������d��N2��NH3��Ũ����Ȳ�һ��ƽ�⣬��ȷ����ѡC��
��5��X����ʽΪ ��
��
��6��������Ϣ�����������Ľṹʽ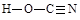
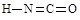 ��
��
���㣺���⿼��ԭ�Ӱ뾶��Ԫ�طǽ����ԣ���ѧ��Ӧ���ʼ��㼰�ṹʽ����д��

W��X��Y��Z��Ԫ�����ڱ���ԭ��ǰ�����ڵ�����Ԫ�أ��й����ǵ���Ϣ���±���ʾ��
| Ԫ�� | �����Ϣ |
| �� | W�Ļ�̬ԭ��L���������K���������3�� |
| �� | X����Ԫ���γɵ�һ�ֻ��������ʹʪ��ĺ�ɫʯ����ֽ���� |
| �� | ����Ϊ����ɫ���壬����������Ӧ��ˮ���ﻯѧʽΪH��O4 |
| �� | Z�Ļ�̬ԭ����Χ�����Ų�ʽΪ(n��1)d10ns1 |
��1��Yλ��Ԫ�����ڱ���________���ڵ�________�壻X�Ļ�̬ԭ�Ӻ�������Ų�ʽ�� ��
��2��W�ļ����Ӱ뾶 X�ļ����Ӱ뾶���>������<����=������Y�ĵ�һ�����ܱ�Z�� �����С������W��X�������̬�⻯���У��е�ϸߵ��� ���ѧʽ����
��3����150������ʱ��������ZW��������Ӧ���ɺ�ɫ��Z2W��ĩ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��4��WԪ���γɵĶ��ֻ���������У����м��Թ��ۼ��ͷǼ��Թ��ۼ��ķ�������Ϊ
����дһ�֣���
��5����25�桢101 kPa�£���֪Z���嵥����Y2��������ȫȼ�պ�ָ���ԭ״̬��ƽ��ÿת��1mol ���ӷ���110.05kJ���÷�Ӧ���Ȼ�ѧ����ʽ�� ��
A��B��C��D��E�� F�����ڱ��ж����ڵ�����Ԫ�أ��й����ʻ�ṹ��Ϣ���±���
| Ԫ�� | �й����ʻ�ṹ��Ϣ |
| A | ����������л���A��һ�ֵ������� |
| B | B������A���ӵ�������ͬ���������������е������Ӱ뾶��С�� |
| C | C��Bͬ���ڣ���������������ԭ�Ӱ뾶���ģ�ϡ��������⣩ |
| D | D������ڻ�ҩ��һ�ֳɷ֣�Ҳ������ɱ������ |
| E | E��Dͬ���ڣ����ڸ�������ԭ�Ӱ뾶��СKs5u |
| F | F���⻯�������������ˮ���ﷴӦ����һ�����ӻ����� |
��1��A��C��ԭ�Ӹ�����Ϊ1��1�γɵĻ�����ĵ���ʽΪ ����0��6 mol�û�����Ͷ�뵽100 mL 3 mol/L BE3��Һ�е����ӷ���ʽΪ �� ��
��2��F���⻯������ �����Ի�Ǽ��ԣ����γɵ� �����Ի�Ǽ��ԣ����ӣ�д��ʵ�����Ʊ����⻯��Ļ�ѧ����ʽ ��
��3����ͼ������ʵ��֤��D��E�ķǽ����Ե�ǿ����
�� ��Һa��b�ֱ�Ϊ �� ��д��ѧʽ����
����Һa�����a��Ӧ�����ӷ���ʽΪ ��
�۷ǽ�����D E������ڻ�С�ڣ������ԭ�ӽṹ�ĽǶȽ���ԭ�� ��