��Ŀ����
��8�֣�ˮú�������Ǻϳɰ���ԭ����Ҳ�Ǻϳ������仯����Ʒ��ԭ�ϡ�
��1��ֱ��ˮú��ȼ�ϵ���У�ͨCO��H2�ļ�Ϊ��ص� ����ѡ�������������������
��2��ˮú���任��Ӧ��CO(g) + H2O(g)  CO2(g) + H2(g) ��H �� 0�����д�ʩ����߷�Ӧ���ʵ��� ��������ѡ��
CO2(g) + H2(g) ��H �� 0�����д�ʩ����߷�Ӧ���ʵ��� ��������ѡ��
a.�����¶� b.������� c.����ѹǿ d.����Ũ��
��3��H2��N2�ڴ��������¸�ѹ�����ºϳɰ��Ļ�ѧ����ʽΪ ��
��4��������ˮ��Һ�����������̵����еĶ������÷�Ӧ�Ļ�ѧ����ʽΪ ��
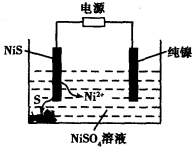
��5������״����582.4L�ϳ�������֪��n(CO)��n(H2)=4��9��ͨ��ϳ�����һ�������¿ɷ���2CO(g)+ 4H2(g) �� CH2=CH2(g)+2H2O(g)��CO(g)+3H2��CH4(g)+H2O(g)����ַ�Ӧ���ⶨ��Ʒ��ֻ�м��顢��ϩ��ˮ�������ٶ�CO��H2����ʣ�ࣩ���Լ����ݳ�����������ϩ�����ʵ������г�������̣���
��1���� ��2��abc ��3��N2 + 3H2 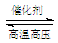 2NH3
2NH3
��4��SO2 + 2NH3 +H2O = (NH4)2SO3 [��SO2 + NH3 +H2O = NH4HSO3]
��5���������⣺n(CO) +n(H2)=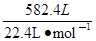 =26mol
=26mol
n(CO)=26mol��4/(4+9)=8mol,n(H2)=26mol��8mol=18mol
�ɷ���ʽ��2CO(g)+ 4H2(g)��C2H4(g)+2H2O(g)��CO(g)+ 3H2(g) �� CH4(g)+ H2O(g)
n(CH4)+2n(C2H4)="8mol" , 3n(CH4)+4n(C2H4)=18mol�����n(C2H4)=3mol��
�����������������ˮú��ȼ�ϵ���У���ԭ��CO��H2����صĸ�����
��ͨ�����¡���ѹ������Ũ�ȡ�����������ܼӿ췴Ӧ���ʣ�
��H2��N2�ڴ��������¸�ѹ�����»��ϳɰ���N2 + 3H2 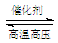 2NH3��ע��÷�ӦΪ���淴Ӧ����
2NH3��ע��÷�ӦΪ���淴Ӧ����
�Ȱ�����ˮ��Һ���ն���������ܲ���(NH4)2SO3��NH4HSO3��
������ο��𰸡�
���㣺����ԭ��ء���Ӧ���ʵ�Ӱ�����ء���ѧ����ʽ����д�뻯ѧ���㡣

 С����ȫ�ܼ��ϵ�д�
С����ȫ�ܼ��ϵ�д�һ�ܱ������г���1mol N2��3mol H2����һ�������·�����ӦN2��3H2 2NH3,�����й�˵����ȷ���ǣ� ��
2NH3,�����й�˵����ȷ���ǣ� ��
| A���ﵽ��ѧƽ��ʱ������Ӧ���淴Ӧ�����ʶ�Ϊ�� |
| B��������3u��(N2)��u��(H2)ʱ����Ӧ�ﵽƽ��״̬ |
| C���ﵽ��ѧƽ��ʱ����λʱ������amolN2��ͬʱ����3amolH2 |
| D����N2��H2��NH3�ķ�������Ϊ1��3��2����Ӧ�ﵽƽ��״̬ |
���á���ѧ����ת�Ʒ����Ʊ�TaS2���壬ij�¶��µ�2L�����ܱ������м���һ���� �� I2��g����TaS2��s���������·�Ӧ
TaS2��s��+2I2��g�� TaI4��g��+S2��g����H��a kJ��mol-1 ��I��
TaI4��g��+S2��g����H��a kJ��mol-1 ��I��
��ƽ��ʱ��TaS2��s����I2��g����TaI4��g������S2��g�������ʵ����ֱ�Ϊ3 mol ��2mol��2mol��2mol��
��1����Ӧ��I����ƽ�ⳣ������ʽK=
��2�������¶��¸�������ijʱ��TaS2��s����I2��g����TaI4��g������S2��g�������ʵ����ֱ�Ϊ2mol��2mol��4mol��4mol�����ʱ��ƽ���� �������Ӧ�����淴Ӧ�����ƶ���v�� v�棨���������������������
��3���ڲ�ͬ�¶��£��÷�Ӧ��ƽ�ⳣ��K���±���
| �¶�/�� | 40 | 80 | 200 |
| ƽ�ⳣ��K | 1 | 1.5 | 4 |
��4��40��ʱ����ú����ܱ������м���2mol I2��g����4mol TaS2��s����I2��g����ƽ��ת����Ϊ (д��������̣��������С�����1λ)
��12 �֣�T��ʱ����ij�ݻ���Ϊ2L�ܱ������г���2molN2��4molH2���ڴ��������·�����Ӧ��N2(g)+3H2(g) 2NH3(g) ��H=��92.0kJ /mol��t0ʱ�̣�����ƽ��������NH3����Ϊ2mol����t1ʱ�̿�ʼ���ı䷴Ӧ��һ����������ϵ�з�Ӧ������ʱ��仯�����������ͼ��ʾ��
2NH3(g) ��H=��92.0kJ /mol��t0ʱ�̣�����ƽ��������NH3����Ϊ2mol����t1ʱ�̿�ʼ���ı䷴Ӧ��һ����������ϵ�з�Ӧ������ʱ��仯�����������ͼ��ʾ��
�Իش��������⣺
��1��T��ʱ��N2��ת����Ϊ��___________���÷�Ӧ��ƽ�ⳣ��K=___________��
��2��T��ʱ��������֤���÷�Ӧ�Ѿ�����ƽ��״̬���ǣ�__________________��
| A����ϵ��ѹǿ���ٸı� |
| B�����������ɫ���ٸı� |
| C��H2�������ٷֺ������ٸı� |
| D��c(N2)��c(NH3)�ı�ֵ���ٸı� |
��4��T��ʱ������ƽ����������м���2molN2��2molNH3�����ʱ v�� ___ v����
����β���ﺬ�е�NO������������ȼ��ȼ�յĸ�����������������Ӧ���£� N2(g)��O2(g)? 2NO(g)����H>0����֪�÷�Ӧ��2 404 �� ʱ��ƽ�ⳣ��K��64��10��4����ش�
2NO(g)����H>0����֪�÷�Ӧ��2 404 �� ʱ��ƽ�ⳣ��K��64��10��4����ش�
��1��ij�¶��£���2L���ܱ������г���N2��O2��1mol��5���Ӻ�O2�����ʵ���Ϊ0.5mol����N2�ķ�Ӧ����________��
��2���ٶ��÷�Ӧ���ں��������½��У��жϸ÷�Ӧ�ﵽƽ��ı�־________��
| A������1 mol N2ͬʱ����1 mol O2 | B����������ܶȲ��� |
| C���������ƽ����Է����������� | D��2v(N2)����v(NO)�� |
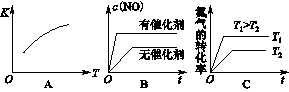
��4������º��ݵ��ܱ������г�������ʵ�����N2��O2���ﵽƽ��״̬���������г���һ����NO�����´ﵽ��ѧƽ��״̬����ԭƽ��״̬��ȣ���ʱƽ��������NO���������______��(������С�����䡱)
��5�����¶��£�ijʱ�̲��������N2��O2��NO��Ũ�ȷֱ�Ϊ2.5��10��1mol/L��4.0��10��2mol/L��3.0��10��3mol/L����ʱ��Ӧ___________(����ڻ�ѧƽ��״̬������������Ӧ������С������淴Ӧ������С�)��������____
(12��).
��1.0 L�ܱ������з���0.10molA(g)����һ���¶Ƚ������·�Ӧ:
A(g) B(g)��C(g) ��H=+85.1kJ��mol��1�����ȷ�Ӧ��
B(g)��C(g) ��H=+85.1kJ��mol��1�����ȷ�Ӧ��
��Ӧʱ��(t)��������������ѹǿ(p)�����ݼ��±���
| ʱ��t/h | 0 | 1 | 2 | 4 | 8 | 16 | 20 | 25 | 30 |
| ��ѹǿp/100kPa | 4.91 | 5.58 | 6.32 | 7.31 | 8.54 | 9.50 | 9.52 | 9.53 | 9.53 |
�ش���������:
��1�������A��ƽ��ת���ʣ�Ӧ��ȡ�Ĵ�ʩΪ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��2������ѹǿP����ʼѹǿP0���㷴Ӧ��A��ת���ʦ�(A)�ı���ʽΪ�ߣߣߣߣߣߣߡ�ƽ��ʱA ��ת����Ϊ�ߣߣߣߣ�.
��3�� ������ѹǿp����ʼѹǿp0��ʾ��Ӧ��ϵ�������ʵ���n���ͷ�Ӧ��A�����ʵ���n��A����
n����_______mol��n��A����_______mol��
���±�Ϊ��Ӧ��AŨ���뷴Ӧʱ������ݣ�����a�� _______________
| ��Ӧʱ��t/h | 0 | 4 | 8 | 16 |
| C��A��/��mol��L-1�� | 0.10 | a | 0.026 | 0.0065 |
�����÷�Ӧ�з�Ӧ���Ũ��c��A���仯��ʱ��������t���Ĺ��ɣ��ó��Ľ����ǣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ��ɴ˹����Ƴ���Ӧ��12hʱ��Ӧ���Ũ��c��A��Ϊ_______mol��L-1
 3C��g��+D��s����2���ӷ�Ӧ�ﵽƽ�⣬��ʱC��Ũ��Ϊ1��2 mol/L��
3C��g��+D��s����2���ӷ�Ӧ�ﵽƽ�⣬��ʱC��Ũ��Ϊ1��2 mol/L�� 

 Ni��CO��4��g��
Ni��CO��4��g��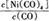 ������?H 0���>����<������
������?H 0���>����<������
 ��
�� )
)