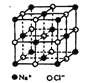
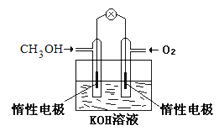
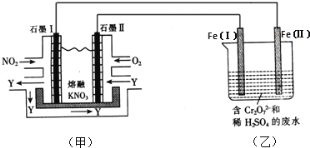
��Ŀ����
����Ŀ����1����ĭ����������ԭ����(�����ӷ���ʽ��ʾ)_____________________��
��2������֪T��ʱ��Kw=1��10-12���ڸ��¶�ʱ����pH=9��NaOH��ҺaL��pH=2��H2SO4��ҺbL���(���Ի�Ϻ���Һ����ı仯)�������û����Һ��pH=3����a��b=____��
��ij��Ԫ�ᣨ��H2A��ʾ����ˮ�еĵ��뷽��ʽ�ǣ�H2A=H++HA����HA- ![]() H++A2������NaHA��Һ�У�д������ԭ�ӣ����ϣ��غ�ĸ�����Ũ�ȹ�ϵ�ĵ�ʽ_____________
H++A2������NaHA��Һ�У�д������ԭ�ӣ����ϣ��غ�ĸ�����Ũ�ȹ�ϵ�ĵ�ʽ_____________
��3��pH��2������һԪ��HA��HB�������Ϊ100 mL��ϡ������pH����Һ����Ĺ�ϵ��ͼ��
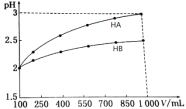
����NaBˮ��Һ�Ƿ�����ԣ�ԭ��______________�������ӷ���ʽ��ʾ����
����NaOH��Һ�ζ�HB��Һ��ָʾ����ѡ��_________���ζ��յ�����Ϊ____________��
A.���� B.��̪ C.ʯ��
��4��NaHSO3��Һ�����ԣ�����Һ�У�������Ũ���ɴ�С��˳��Ϊ____________��
���𰸡�3HCO3-+Al3+=3CO2��+Al(OH)3�� 9:2 c(Na+)=c(HA-)+c(A2-) B-+H2O![]() HB+OH- B ���������һ��NaOH��Һʱ��ƿ����Һ��죬��30���ڲ��ָ�ԭɫ�� c(Na+)>c(HSO3-)>c(H+)>c(SO32-)>c(OH-)
HB+OH- B ���������һ��NaOH��Һʱ��ƿ����Һ��죬��30���ڲ��ָ�ԭɫ�� c(Na+)>c(HSO3-)>c(H+)>c(SO32-)>c(OH-)
��������
(1)��ĭ�������ԭ����NaHCO3��Һ��Al2(SO4)3��Һ��NaHCO3ˮ�ⷴӦ��HCO3-+H2O![]() H2CO3+OH-��Al2(SO4)3ˮ�ⷴӦ��Al3++3H2O
H2CO3+OH-��Al2(SO4)3ˮ�ⷴӦ��Al3++3H2O![]() Al(OH)3+3H+���ɴ˷�����
Al(OH)3+3H+���ɴ˷�����
(2) ��ǿ����ǿ���Ϻ���Һ������ԣ�ȡ�������H+����OH-�����ʵ�������Դ�С�����ݷ�ӦH++OH-=H2O��֪����n(H+)>n(OH-)�������Һ�����ԣ���n(H+)=n(OH-)�������Һ�����ԣ���n(H+)<n(OH-)�������Һ�Լ��ԡ��ڸ��ݵ��뷽��ʽ��H2A=H++HA����HA- ![]() H++A2��֪��HA-����ˮ�⣬NaHA��Һ����H2A���ӡ��ɴ˷�����
H++A2��֪��HA-����ˮ�⣬NaHA��Һ����H2A���ӡ��ɴ˷�����
(3) ��ͼ���֪HA��HB����Һ��ϡ����10����HA��Һ��pH��2���ӵ�3����c(H+)��10-2mol/L��С��10-3mol/L����H+Ũ�ȼ�С��ԭ����![]() ��˵��HA��ǿ���HB��Һϡ�ͺ�c(H+)>10-3mol/L��˵��HB��Һ�д��ڵ���ƽ���ƶ�������֪HB�����ᡣNaB����ǿ�������Σ��к͵ζ���ָʾ����ѡ��Ҫ��ѭ��ͼ�ǡ�÷�Ӧʱ��Һ�������ָʾ��������ɫ�仯��pH��Χ��һ�£��ɴ˷������
��˵��HA��ǿ���HB��Һϡ�ͺ�c(H+)>10-3mol/L��˵��HB��Һ�д��ڵ���ƽ���ƶ�������֪HB�����ᡣNaB����ǿ�������Σ��к͵ζ���ָʾ����ѡ��Ҫ��ѭ��ͼ�ǡ�÷�Ӧʱ��Һ�������ָʾ��������ɫ�仯��pH��Χ��һ�£��ɴ˷������
(4) ��ΪH2SO3������ǿ�ᣬ����NaHSO3��ˮ��Һ�м��е���ƽ�⣺HSO3-![]() H++SO32-��Ҳ��ˮ��ƽ�⣺HSO3-+H2O
H++SO32-��Ҳ��ˮ��ƽ�⣺HSO3-+H2O![]() H2SO3+OH-���ٽ�����������
H2SO3+OH-���ٽ�����������
(1)��ĭ�������ԭ����NaHCO3��Һ��Al2(SO4)3��Һ��HCO3-��Al3+������ٽ���ˮ�ⷴӦ���ܽ��е��ף����ʱ����������CO2��ĭ�Դﵽ����Ŀ�ġ��䷴Ӧԭ��Ϊ3HCO3-+Al3+=3CO2��+Al(OH)3����
(2)����T��ʱKw=1��10-12��֪�����¶���������Һc(H+)=c(OH-)=![]() =
=![]() =10-6mol/L����������Һ��pH=6����pH=9��NaOH��Һ��c(OH-)=
=10-6mol/L����������Һ��pH=6����pH=9��NaOH��Һ��c(OH-)=![]() =
=![]() =10-3mol/L��pH=2��H2SO4��Һ��c(H+)=10-2mol/L��a LNaOH��Һ��n(OH-)=10-3a mol��b LH2SO4��Һ��n(H+)=0.01b mol����Ϊ���û����Һ��pH=3<6��������Һ�����ԣ�˵��H2SO4��������c(H+)��=10-3mol/L������c(H+)��=
=10-3mol/L��pH=2��H2SO4��Һ��c(H+)=10-2mol/L��a LNaOH��Һ��n(OH-)=10-3a mol��b LH2SO4��Һ��n(H+)=0.01b mol����Ϊ���û����Һ��pH=3<6��������Һ�����ԣ�˵��H2SO4��������c(H+)��=10-3mol/L������c(H+)��=![]() =10-3mol/L�����a:b=9:2��
=10-3mol/L�����a:b=9:2��
����Ϊ��Ԫ��H2A�ĵ�һ��������ȫ���ڶ���ֻ�в��ֵ��룬��HA-����Һ��ֻ�ܲ��ֵ��������ˮ�⣺HA-![]() H++A-������NaHA��Һ�������غ��ʽΪc(Na+)=c(HA-)+c(A2-)��
H++A-������NaHA��Һ�������غ��ʽΪc(Na+)=c(HA-)+c(A2-)��
(3) ��ͼ���֪HA��HB����Һ��ϡ����10����HA��Һ��pH��2���ӵ�3����c(H+)��10-2mol/L��С��10-3mol/L����H+Ũ�ȼ�С��ԭ����![]() ��˵��HA��ǿ���HB��Һϡ�ͺ�c(H+)>10-3mol/L��˵��HB��Һ�д��ڵ���ƽ���ƶ���˵��HB�����ᡣ����ΪHB�����ᣬ�������ӦNaBΪǿ�������Σ���Һ�ʼ��ԣ�ԭ��������ӷ���ʽ��ʾ��B-+H2O
��˵��HA��ǿ���HB��Һϡ�ͺ�c(H+)>10-3mol/L��˵��HB��Һ�д��ڵ���ƽ���ƶ���˵��HB�����ᡣ����ΪHB�����ᣬ�������ӦNaBΪǿ�������Σ���Һ�ʼ��ԣ�ԭ��������ӷ���ʽ��ʾ��B-+H2O![]() HB+OH-������ΪNaOH��HBǡ���к�ʱ����NaB������Һ�Լ��ԣ�Ӧѡ���ڼ��Է�Χ�ڱ�ɫ��ָʾ��������ָʾ����ѡ���̪����ѡB���ζ��յ�����Ϊ�����������һ��NaOH��Һʱ��ƿ����Һ��죬��30���ڲ��ָ�ԭɫ��
HB+OH-������ΪNaOH��HBǡ���к�ʱ����NaB������Һ�Լ��ԣ�Ӧѡ���ڼ��Է�Χ�ڱ�ɫ��ָʾ��������ָʾ����ѡ���̪����ѡB���ζ��յ�����Ϊ�����������һ��NaOH��Һʱ��ƿ����Һ��죬��30���ڲ��ָ�ԭɫ��
(4)��ΪH2SO3������ǿ�ᣬ����NaHSO3��ˮ��Һ�м��е���ƽ�⣺HSO3-![]() H++SO32-��Ҳ��ˮ��ƽ�⣺HSO3-+H2O
H++SO32-��Ҳ��ˮ��ƽ�⣺HSO3-+H2O![]() H2SO3+OH-������ΪNaHSO3��Һ�����ԣ�����HSO3-�ĵ���̶ȴ�����ˮ��̶ȣ�һ�����˵����̶Ⱥ�ˮ��̶ȶ����������NaHSO3��Һ������Ũ���ɴ�С��˳��Ϊc(Na+)>c(HSO3-)>c(H+)>c(SO32-)>c(OH-)��
H2SO3+OH-������ΪNaHSO3��Һ�����ԣ�����HSO3-�ĵ���̶ȴ�����ˮ��̶ȣ�һ�����˵����̶Ⱥ�ˮ��̶ȶ����������NaHSO3��Һ������Ũ���ɴ�С��˳��Ϊc(Na+)>c(HSO3-)>c(H+)>c(SO32-)>c(OH-)��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�