��Ŀ����
����Ŀ������26����ʼ���������¶��壬�����ӵ�������NA������ȷֵ6.02214076��1023������˵����ȷ����
A. 18 gT2O�к��е�������Ϊ12NA
B. ��23.5gAgI��ˮ�ƳɵĽ����н���������ĿΪ0.1NA
C. ��״���£�2.24LCl2ȫ������ˮ������Һ�е�Cl����ĿΪ0.1NA
D. 1molij����CnH2n+2��n��1�������к��еĹ��ۼ���Ϊ(3n+1)NA
���𰸡�D
��������
A��18gT2O�����ʵ���Ϊ![]() =
=![]() mol��
mol��![]() mol��ˮ�����к������ӵ����ʵ���Ϊ
mol��ˮ�����к������ӵ����ʵ���Ϊ![]() mol��12=
mol��12=![]() mol����A����
mol����A����
B�����������ǵ����ķ��ӣ����Ƕ��AgI�ľۺ��壬�����������23.5gAgI��ˮ�ƳɵĽ����н�������������B����
C��������ˮ�ķ�ӦΪ���淴Ӧ�����ܽ��г��ף������ɵ���HCl��HClO������Һ�е������Ӹ���С��0.1NA������C����
D��1molCnH2n+2(����)�к���(n-1)mol̼̼����(2n+2)mol̼������ܹ�����(3n+1)mol���ۼ������й��ۼ���Ϊ(3n+1)NA����D��ȷ��
��ѡD��

 ����˼ά�żӿ���ϵ�д�
����˼ά�żӿ���ϵ�д� �����Ծ�ϵ�д�
�����Ծ�ϵ�д� �ο�����������100��ϵ�д�
�ο�����������100��ϵ�д�����Ŀ�������ڼ��������¿ɻ�ԭ����ͭ����������ˮ�����⣬����̼�������ij��ѧС��������ͼװ��̽���䷴Ӧ���
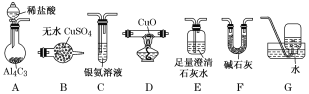
[��������]��CO����������Һ��Ӧ��CO��2[Ag(NH3)2]����2OH��===2Ag����2NH4+��CO32����2NH3��
��Cu2OΪ��ɫ������Ag+��Ӧ���ܷ�����Ӧ��Cu2O��2H��===Cu2+��Cu��H2O��
��1��װ��A�з�Ӧ�Ļ�ѧ����ʽΪ___________________________________________��
��2�������������װ�ô����ҵ�����˳��ΪA��__________________��(����ĸ���)
��3��ʵ���еμ�ϡ����IJ���Ϊ______________________________________________��
��4����֪��������к���CO����װ��C�пɹ۲쵽��������________________��װ��F������Ϊ_________________________________________��
��5������Ӧ������װ��D���Թ��й���ȫ����Ϊ��ɫ��
�����ʵ��֤����ɫ�����к���Cu2O��______________________________________________��
����֤����ɫ�������Ƿ���Cu����ͬѧ�������ʵ�飺��������ɫ�����м�������0.1mol��L1AgNO3��Һ��������Һ�������ݴ��жϺ�ɫ�����к���Cu����ͬѧ��Ϊ�÷�������������֤����ͬѧ�Ľ��ۣ������������¶Ա�ʵ�飬��ɱ������ݡ�
ʵ�鲽��(��Ҫ��д�������������) | Ԥ������ͽ��� |
__________________ | ���۲쵽��Һ����������֤����ɫ�����к���Cu�����۲쵽��Һ����������֤����ɫ�����к���Cu |


