��Ŀ����
5���������ͬ����Ԫ�أ�������������Ϳ����о��й㷺��Ӧ�ã������Ǽ��ֳ��������| ��þ�� | ��ɰ | ���� | ƫ������ |
| Mg2B2O5•H2O | Na2B4O7•10H2O | H3BO3 | NaBO2 |
��1��д����Ԫ�������ڱ��е�λ�õڶ����ڢ�A�壮
��2����ҵ������þ��Ϊԭ����ȡ�𣬸ù��յ��м���̻ᷢ����Ӧ��B2O3+3Mg=2B+3MgO����ÿ����1mol����ת�Ƶĵ�����Ϊ3NA��
��3����ҵ�ϳ��ý�����ұ�����۽�������д����������������V2O5������Ϊԭ�ϻ�ý������Ļ�ѧ����ʽ3V2O5+10Al$\frac{\underline{\;����\;}}{\;}$6V+5Al2O3��
��4��������һ��һԪ���ᣬ����ˮ����ʱ���ˮ�����OH-���ͷų�ˮ�����H+��Ƥ���ϲ�С����������������Һ��һ�����ô���ˮ��ϴ��Ȼ����Ϳ��������Һ����д���������������Ʒ�Ӧ�����ӷ���ʽH3BO3+OH-=[B��OH��4]-��
��5�����⻯�ƣ�NaBH4����һ��ǿ��ԭ������ˮ���ҷ�Ӧ�����������壬��д���÷�Ӧ�Ļ�ѧ����ʽNaBH4+2H2O�TNaBO2+4H2����
��6��ƫ������������ˮ������Һ�Լ��ԣ�����ԡ��������ԡ����ԡ�����ԭ����BO2-+2H2O?H3BO3+OH-�������ӷ���ʽ��ʾ��
���� ��1����Ԫ�غ���������Ӳ㣬������������ӣ�
��2���ɷ���ʽ��֪��B2O3+3Mg=2B+3MgO������2mol����ת��6mol�ĵ��ӣ�
��3�����ȷ�Ӧ��������ԭ���������������������������ݴ˵��ӵ�ʧ��д����ʽ��
��4��������һ��һԪ���ᣬ�����������Ʒ�Ӧ��
��5�����⻯�ƣ�NaBH4�����л���ѧ�е�һ�ֳ��û�ԭ��������ˮ��ˮ�����������ƺ��������ݴ���д����ʽ��
��6��ƫ��������ǿ�������Σ�ˮ��ʼ��ԣ�����ʽΪ��BO2-+2H2O?H3BO3+OH-��
��� �⣺��1����Ԫ�غ���������Ӳ㣬������������ӣ�������Ԫ�������ڱ��е�λ��Ϊ���ڶ����ڢ�A�壬�ʴ�Ϊ���ڶ����ڢ�A�壻
��2���ɷ���ʽ��֪��B2O3+3Mg=2B+3MgO������2mol����ת��6mol�ĵ��ӣ���������1mol����ת��3mol�ĵ��ӣ���ת�Ƶ�����Ϊ3NA���ʴ�Ϊ��3NA��
��3�����ȷ�Ӧ��������ԭ������������������������������ʽΪ��3V2O5+10Al$\frac{\underline{\;����\;}}{\;}$6V+5Al2O3���ʴ�Ϊ��3V2O5+10Al$\frac{\underline{\;����\;}}{\;}$6V+5Al2O3��
��4��������һ��һԪ���ᣬ�����������Ʒ�Ӧ���䷴Ӧ����ʽΪ H3BO3+NaOH=Na[B��OH��4]���������ӷ���ʽΪ H3BO3+OH-=[B��OH��4]-���ʴ�Ϊ��H3BO3+OH-=[B��OH��4]-��
��5�����⻯�ƣ�NaBH4�����л���ѧ�е�һ�ֳ��û�ԭ��������ˮ��ˮ�����������ƺ���������Ӧ����ʽΪ��NaBH4+4H2O�TNa[B��OH��4]+4H2����NaBH4+2H2O�TNaBO2+4H2�����ʴ�Ϊ��NaBH4+4H2O�TNa[B��OH��4]+4H2����NaBH4+2H2O�TNaBO2+4H2����
��6��ƫ��������ǿ�������Σ�ˮ��ʼ��ԣ�����ʽΪ��BO2-+2H2O?H3BO3+OH-���ʴ�Ϊ�����ԣ�BO2-+2H2O?H3BO3+OH-��
���� ���⿼������Ԫ�صĻ���������ʣ���Ŀ�Ѷ��еȣ�ע�������Ŀ�е���Ϣ������

 ȫ�ų��100��ϵ�д�
ȫ�ų��100��ϵ�д� Ӣ�ŵ��ϵ�д�
Ӣ�ŵ��ϵ�д�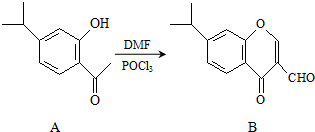 �л���A��B��Ϊ�ϳ�ij�ֿ�֧��������ҩ����м��壬A��һ�������¿�ת��ΪB����ͼ��ʾ��������˵����ȷ���ǣ�������
�л���A��B��Ϊ�ϳ�ij�ֿ�֧��������ҩ����м��壬A��һ�������¿�ת��ΪB����ͼ��ʾ��������˵����ȷ���ǣ�������| A�� | ����A������̼ԭ�Ӿ�λ��ͬһƽ�� | |
| B�� | ��FeCl3��Һ�ɼ�������B���Ƿ����A | |
| C�� | ����B���ܷ���������Ӧ�����ܷ���ˮ�ⷴӦ | |
| D�� | 1mol B������5mol H2�����ӳɷ�Ӧ |
| A�� | ��Һһ�������� | B�� | ��Һ��һ�����ڣ�Y2-+H2O?OH-+HY- | ||
| C�� | ��Һ��ˮϡ�ͣ�����Ũ�ȶ���С | D�� | ��Һ�У�c��Na+����c��Y2-�� |
| A�� | ���������ޱ������ӷ�Ӧ��CO2+OH-=HCO3- | |
| B�� | �����������ֹ������ķ�Ӧ��Al3++3HCO3-=Al��OH��3+3CO2�� | |
| C�� | ���������й�������������ѧ��Ӧ���ҷ�Ӧ���������Һ�ʼ��� | |
| D�� | ����CO2��ΪNH3��ŨNaOH��Һ��Ϊˮ��������Ҳ������ȱ��ĵ����� |
| A�� | ����Һ���������Եõ�4 mol NaCl | |
| B�� | ����Һ���ɲ����գ��õ��Ĺ��������NaCl��NaBr��Na2SO4 | |
| C�� | ����Һ�еμ�KI������Һ����Һ������������һ������ | |
| D�� | ��ͨ�����������Ϊ11.2 L����״��������Ӧ�����ӷ���ʽΪ��2I-+Cl2=I2+2C1- |
| A�� | �����к�ǿ�Ŀ���ʴ����������Ϊ�䲻��������������Ӧ | |
| B�� | ������ë������ȼ�պ����ɶ�����̼��ˮ | |
| C�� | ����ɻ��ϵ��մɷ���Ƭ�����������ǽ������� | |
| D�� | ʳƷ���rĤ������ˮ����������Ʒ����Ҫ�ɷ��Ǿ�����ϩ |
| ѡ�� | ������ | �����Լ�����Һ�� | �����Լ�������Ӧ�����ӷ���ʽ |
| A | K+��Fe3+��NO3-��SO42- | ����KSCN | Fe3++3SCN-=Fe��SCN��3 |
| B | Na+��AlO2-��Cl-��OH- | ����NaHCO3 | OH-+HCO3-=CO32-+H2O |
| C | H+��Ba2+��Al3+��HCO3- | ����NaOH | Al3++3OH-=Al��OH��3�� |
| D | K+��Na+��S2O32-��Cl- | �������� | 2S2O32-+2H+=SO42-+3S��+H2O2 |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | I A��͢�A��Ԫ�ؼ���γɹ��ۻ���������ӻ����� | |
| B�� | ��H2SO3�����Ա�H2CO3ǿ������S�ķǽ����Ա�Cǿ | |
| C�� | H2O2��CH3CH3��Cl-��K+�еĵ����������������ֱ���� | |
| D�� | Na+��Al3+��O2-��F-�İ뾶��С |
