��Ŀ����
20���±�Ϊ��ʽ���ڱ���һ���֣����еı�Ŵ�����Ӧ��Ԫ�أ�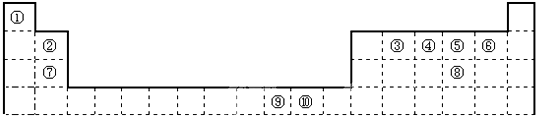
��1��д���ϱ���Ԫ�آ��̬ԭ�ӵ���Χ�����Ų�ͼ1s22s22p63s23p63d104s1��
��2����Ԫ�آ�����γɵ�ˮ����������廯�����У�Ԫ�آ۵��ӻ���ʽΪ��sp2��
��3����Ҫ��������и��⣺
a����Ԫ�آ����γɵĵ��ʻ�Ϊ�ȵ�����ķ��ӡ����ӵĻ�ѧʽCO��C22-��CN-��NO+��O22+����һ�֣���
b��Ԫ�آܵ���̬�⻯��X��ˮ��Һ�����ӹ�ҵ�У�������ʴ��H2O2�����������������Ӧ�IJ��ﲻ��Ⱦ�������仯ѧ����ʽΪ2NH3+3H2O2=N2+6H2O��
��4���ڲⶨ������γɻ��������Է�������ʱ��ʵ���õ�ֵһ���������ֵ����Ҫԭ���ǣ�HF����֮�����γ�������γɣ�HF��n�Ϸ��ӣ�ʵ�ʲ�õ�ΪHF�ͣ�HF��n����Է���������ƽ��ֵ����HF�ķ�������
��5��Ԫ�آ����Ԫ�ؾ����γ�+2�����������ṹ���;����Ȼ�蘆���ͬ��
a���Ƚ����Ӱ뾶��С��Cl-����Cs+������ڡ�����С�ڡ����ڡ�����
b��Ԫ�آ������+2�����Ӱ뾶�ֱ�Ϊ69pm��78pm����Ƚ�������������۵�ϸߵ�ΪNiO���ѧʽ���������ԭ�������������е��������ӵĵ�ɣ�����ֵ������ȣ�Ni2+�İ뾶С��NiO���������Ӻ˼��С��FeO���������Ӻ˼�࣬��֪NiO�ľ����ܽϴ��۵�ߣ�
��6��Ԫ�آ����γɵĵ��ʾ�����ԭ�ӵĶѻ���ʽΪfcc�������������������߳�Ϊ361pm����֪�ⵥ�ʵ��ܶ�Ϊ9.00g•cm-3��ԭ����Ϊ63.6����ش��������⣺
a�������и�ԭ�ӵ���λ��Ϊ12��һ�������а�����ԭ����ĿΪ4��
b���ⵥ�ʵľ����������4.70��10-23cm3�������ӵ�����Ϊ$\frac{4��63.6}{9.00��4.70��10{\;}^{-23}}$/mol=6.01��1023mol-1����ʽ���㣩��
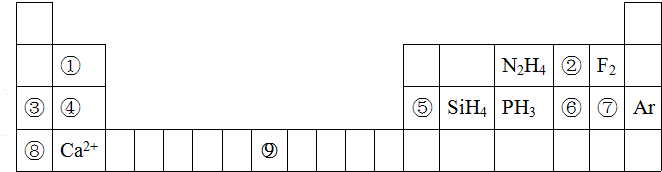
���� ��Ԫ�������ڱ��е�λ�ÿ�֪��ΪH����ΪLi����ΪC����ΪN����ΪO����ΪF����ΪMg����ΪS����ΪNi����ΪCu��
��1����ΪCu��ԭ������Ϊ29����Ϲ���ԭ����д�����Ų�ʽ��
��2��Ԫ�آ�����γɵ�ˮ�������ΪC2H4�������γɵĦļ��жϣ�
��3��Ԫ�آ����γɵĵ���ΪN2������14�����ӣ�Ԫ�آܵ���̬�⻯��XΪNH3����H2O2��Ӧ����N2��ˮ��
��4��HF����֮�����γ�������γɣ�HF��n�Ϸ��ӣ�
��5��a����������Ų���ͬ�����ӣ��˵����Խ�����Ӱ뾶ԽС��
b�����Ӱ뾶ԽС�������۵�Խ�ߣ�
��6�����þ�̯������
��� �⣺��Ԫ�������ڱ��е�λ�ÿ�֪��ΪH����ΪLi����ΪC����ΪN����ΪO����ΪF����ΪMg����ΪS����ΪNi����ΪCu��
��1����ΪCu��ԭ������Ϊ29�������Ų�ʽΪ1s22s22p63s23p63d104s1��
�ʴ�Ϊ��1s22s22p63s23p63d104s1��
��2��Ԫ�آ�����γɵ�ˮ�������ΪC2H4���ṹ��ʽΪCH2=CH2��C�γ�3���ļ���Ϊsp2�ӻ���
�ʴ�Ϊ��sp2��
��3��a��Ԫ�آ����γɵĵ���ΪN2������14�����ӣ���Ԫ�آ����γɵĵ��ʻ�Ϊ�ȵ�����ķ��ӡ�������CO��C22-��CN-��NO+��O22+��
�ʴ�Ϊ��CO��C22-��CN-��NO+��O22+��
b��Ԫ�آܵ���̬�⻯��XΪNH3����H2O2��Ӧ����N2��ˮ������ʽΪ2NH3+3H2O2=N2+6H2O��
�ʴ�Ϊ��2NH3+3H2O2=N2+6H2O��
��4��F�ķǽ����Ժ�ǿ����Ӧ�⻯���к��������HF����֮�����γ�������γɣ�HF��n�Ϸ��ӣ�ʵ�ʲ�õ�ΪHF�ͣ�HF��n����Է���������ƽ��ֵ����HF�ķ�������
�ʴ�Ϊ��HF����֮�����γ�������γɣ�HF��n�Ϸ��ӣ�ʵ�ʲ�õ�ΪHF�ͣ�HF��n����Է���������ƽ��ֵ����HF�ķ�������
��5��a��Cl-��K+��������Ų���ͬ���˵����Խ�����Ӱ뾶ԽС��Cl��ԭ������С��K����Cl-���Ӱ뾶�ϴ�
�ʴ�Ϊ�����ڣ�
b�������������е��������ӵĵ�ɣ�����ֵ������ȣ����Ӱ뾶ԽС��������Խ�������۵�Խ�ߣ�
�ʴ�Ϊ��NiO�������������е��������ӵĵ�ɣ�����ֵ������ȣ�Ni2+�İ뾶С��NiO���������Ӻ˼��С��FeO���������Ӻ˼�࣬��֪NiO�ľ����ܽϴ��۵�ߣ�
��6��a��Cu���ʵľ���Ϊ�����������ܶѻ����Զ���Cuԭ���о�����֮�����Cuԭ�Ӵ��������ϣ�ÿ������Ϊ12���湲�ã���Cu����λ��Ϊ12��
һ�������а�����ԭ����ĿΪ6��$\frac{1}{2}$+$\frac{1}{8}$��8=4��
�ʴ�Ϊ��12��4��
b�������߳�Ϊ361pm���������Ϊ��361��10-10cm��3=4.70��10-23cm3��
�谢���ӵ�����ΪNA��
���=$\frac{m}{V}$=$\frac{\frac{4��63.6}{{N}_{A}}}{4.70��1{0}^{-23}}$=9.00g•cm-3����NA=$\frac{4��63.6}{9.00��4.70��10{\;}^{-23}}$/mol��
�ʴ�Ϊ��4.70��10-23��NA=$\frac{4��63.6}{9.00��4.70��10{\;}^{-23}}$/mol=6.01��1023mol-1��
���� ������Ԫ���ƶ�Ϊ���壬�ۺϿ������ʽṹ�����ʣ��漰��������Ų����ɡ��ӻ���������ӽṹ���������������ȣ��Ѷ��еȣ���6��ע�����þ�̯�����о������㣮

 Сѧ���AB��ϵ�д�
Сѧ���AB��ϵ�д� ABC����ȫ�ž�ϵ�д�
ABC����ȫ�ž�ϵ�д�| A�� | ������ | B�� | ���� | C�� | ��֬ | D�� | ���� |
| A�� | ��������SO42-Ũ�������� | |
| B�� | ����ͨ��������ͭƬ����пƬ | |
| C�� | ������ӦʽΪZn-2e-�TZn2+ | |
| D�� | ��ԭ��ع��������е������Һ��pHֵ���� |
| A�� | �������� | B�� | ��-2-����ϩ | C�� | �����嶡�� | D�� | �����ᱽ���� |
| A�� | ���õķ�ɢϵ��������Һ | |
| B�� | ���õķ�ɢϵ�з�ɢ��ΪFe2O3 | |
| C�� | �÷�ɢϵ�ܲ��������ЧӦ | |
| D�� | �ô��������Fe��OH��2��Fe��OH��3������Һ |
 ��
��