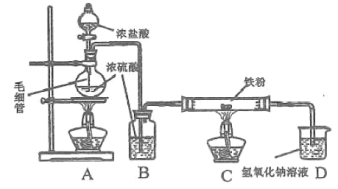
��Ŀ����
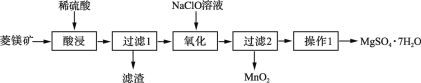
����Ŀ��MgSO4��7H2O��һ����Ҫ�Ļ���ԭ�ϣ�ij�о���ѧϰС�������������þ��ʯ(��Ҫ�ɷ���MgCO3��������MnCO3��SiO2)��ȡMgSO4��7H2O��ʵ���������£�
(1)����1��������Ҫ�ɷ���____��
(2)����ʱ������Ӧ�����ӷ�Ӧ����ʽΪ________________��
(3)����1Ҫ����____��____�����ˡ�ϴ�ӡ�����Ȳ���ſɵõ���Ʒ��
(4)ijMgSO4��7H2O��Ʒ�л�������CaSO4��Al2(SO4)3��Fe2(SO4)3����ͨ�����з����ⶨ�䴿��:
��.��ȡ������Ʒ10.00 g����ˮ�ܽ���� 250 mL��ҺA���á�
��.ȡ10 .00 mL��ҺA���������Ҵ�����Һ������������ˮ����pH��7~8������0.02 mol��L-1 EDTA(H2Y2-)��֮��ַ�Ӧ������ȥEDTA��Һ84.00 mL��
��.ȡ25.00 mL��ҺA���������Ҵ�����Һ���ټ���0.125 mol��L-1 NaOH��Һ����pH��12~13����0.02 mol��L-1 EDTA��֮��ַ�Ӧ������ȥEDTA��Һ10.00 mL��
��֪:
�� Mg2+��pH=9.1ʱ��ʼ������pH=12.1ʱ������ȫ��Ca2+��pH=13.1ʱ��δ������
�� Mg2++H2Y2-=MgH2Y��Ca2++H2Y2-=CaH2Y��
�� �����Ҵ������������������ʲ���EDTA��Ӧ��
������Ʒ��MgSO4��7H2O����������__________(д���������)��
���𰸡�SiO2 Mn2++ClO+H2O�TMnO2��+Cl+2H+ ����Ũ�� ��ȴ�ᾧ 98.4%
��������
��þ��ʯ(��Ҫ�ɷ���MgCO3��������MnCO3��SiO2)��ϡ�����ȡ������1�õ���ҺΪ�����̡�����þ��Һ������1������ΪSiO2����Һ�м���NaClO��Һ������ӦMn2++ClO-+H2O�TMnO2��+Cl-+2H+����Mn2+������MnO2����������2������ҺΪMgSO4���Ȼ����Һ������Һͨ������1(����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ������)��MgSO47H2O���壬�Դ˽����⡣
(1)��������У�ֻ��SiO2����ϡ���ᷴӦ��������1ΪSiO2��
(2)�����Ʊ����̿�֪������NaClO��Һ��Mn2+������MnO2����,�÷�Ӧ�����ӷ���ʽΪ��Mn2++ClO+H2O�TMnO2��+Cl+2H+��
(3)����1������þ���Ȼ����Һ�еõ�MgSO47H2O���壬��������Ϊ������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����ȣ�����Ϊ�Ȼ�泥�ͨ������ϴ��Һ���Ƿ��������Ӽ����жϲ�Ʒ�Ƿ�ϴ�Ӹɾ�����������Ϊ��ȡ���һ��ϴ��Һ���μӼ��������ữ����������Һ�����ް�ɫ���ǣ���֤����ϴ�Ӹɾ���
(4)�ɵڢ�֪��10mL��ҺA����n(EDTA)=0.02molL1��0.084L=0.00168mol����n(Mg2+)+n(Ca2+)=0.00168mol����25mL��ҺA�к���Mg2+��Ca2+�������ʵ���Ϊ��0.00168mol��![]() =4.2��103mol���ɵڢ�֪��25mL��ҺA�У�
=4.2��103mol���ɵڢ�֪��25mL��ҺA�У�
![]()
n(MgSO47H2O)=4.2��103mol2��104mol=4��103mol��250 mL��ҺA��n(MgSO47H2O)= 4��103mol��![]() =4��102mol��,����Ʒ��MgSO47H2O����������Ϊ��
=4��102mol��,����Ʒ��MgSO47H2O����������Ϊ��![]() ��100%=98.4%��
��100%=98.4%��

 �ܿ�����ĩ��̾�ϵ�д�
�ܿ�����ĩ��̾�ϵ�д�����Ŀ����ҵ����﮻�ʯΪԭ������̼��﮵IJ��ֹ�ҵ�������£�
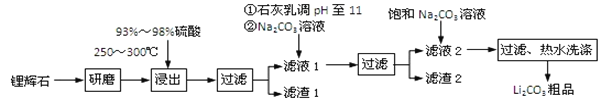
��֪��
��﮻�ʯ����Ҫ�ɷ�ΪLi2O��Al2O3��4SiO2�����к�����Ca��MgԪ�ء�
��Li2O��Al2O3��4SiO2 + H2SO4��Ũ�� ![]() Li2SO4 + Al2O3��4SiO2��H2O
Li2SO4 + Al2O3��4SiO2��H2O
��ijЩ���ʵ��ܽ�ȣ�s�����±���ʾ��
T/�� | 20 | 40 | 60 | 80 |
s��Li2CO3��/g | 1.33 | 1.17 | 1.01 | 0.85 |
s��Li2SO4��/g | 34.2 | 32.8 | 31.9 | 30.7 |
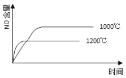
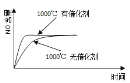
��1�����������з����Al2O3����������ͼ��ʾ����д�����ɳ��������ӷ���ʽ______��
![]()
��2����֪����2����Ҫ�ɷ���Mg��OH��2��CaCO3������Һ1�м���ʯ����������ǣ����û�ѧƽ��ԭ��������________________________________________________��
��3�����һ�������У�������ˮϴ������ԭ����______________________________��
��4����ҵ�ϣ���Li2CO3��Ʒ�Ʊ��ɸߴ�Li2CO3�IJ��ֹ������£�
a.��Li2CO3�������������۵�����Һ��LiOH��Һ������Һ������������ѡ����Ĥ�������ö��Ե缫��⡣
b.������LiOH��Һ�м�������NH4HCO3��Һ�����ȣ����ˡ���ɵøߴ�Li2CO3��
��a�У������ĵ缫��Ӧʽ��_________________________
�ڵ���LiOH��ҺŨ�������ԭ��_________________��b������Li2CO3��Ӧ�Ļ�ѧ����ʽ��___________________________________________��
��5����������﮵���ܷ�ӦΪ��FePO4+Li![]() LiFePO4������еĹ������ʿɴ���Li+����д���õ�طŵ�ʱ��������Ӧ��__________________��
LiFePO4������еĹ������ʿɴ���Li+����д���õ�طŵ�ʱ��������Ӧ��__________________��