��Ŀ����
����Ŀ��ij��ɫ���ʼ�ֻ������Ԫ�أ�Ϊ̽�����ʼ���ɺ����ʣ���Ʋ��������ʵ�顣������̬�⻯�����ڱ���µ��ܶ�Ϊ 1.518 g��L-1����������ͬԪ�صĻ��ϼ���ͬ��
��1�������Ԫ��_____________��
��2��д����������Ũ���ᷴӦ�����ӷ���ʽ______________��
��3���������ڹ��������г��ȼ�պ��ٽ��������ͨ��BaCl2��Һ�����ְ�ɫ������д���ù������ܷ�Ӧ�����ӷ���ʽ_________________ ��
���𰸡�Fe��S Fe3S4 +6H+=3Fe2++S��+H2S�� 2SO2+O2+2H2O+2Ba2+=2BaSO4��+4H+
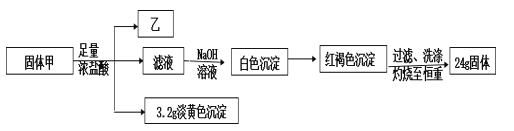
��������
��������Ũ���ᷴӦ�õ�����Һ�м�������NaOH��Һ��������ɫ��������ɫ������Ϊ���ɫ����Һ�к�Fe2+�����ɫ����ΪFe(OH)3�����ɫFe(OH)3���պ�õ�24g�Ĺ���ΪFe2O3������Fe�غ㣬���к�FeԪ�أ���n(Fe)=2n(Fe2O3)=![]() 2=0.3mol����+Ũ����
2=0.3mol����+Ũ����![]() ��̬�⻯����+��Һ+����ɫ����������ֻ������Ԫ�أ�M(��)=1.518g/L
��̬�⻯����+��Һ+����ɫ����������ֻ������Ԫ�أ�M(��)=1.518g/L![]() 22.4L/mol��34g/mol����ΪH2S������ɫ����ΪS��n(S)=
22.4L/mol��34g/mol����ΪH2S������ɫ����ΪS��n(S)=![]() =0.1mol�����к�SԪ�أ�������Ԫ�صĻ��ϼ�Ϊ-2�ۣ���������ͬԪ�صĻ��ϼ���ͬ��������Ԫ�صĻ��ϼ�Ϊ-2�ۣ�����Ũ����ķ�ӦΪ������ԭ��Ӧ�������FeԪ�ص�ƽ�����ϼ�Ϊx�����ݵ�ʧ�����غ㣬0.3mol
=0.1mol�����к�SԪ�أ�������Ԫ�صĻ��ϼ�Ϊ-2�ۣ���������ͬԪ�صĻ��ϼ���ͬ��������Ԫ�صĻ��ϼ�Ϊ-2�ۣ�����Ũ����ķ�ӦΪ������ԭ��Ӧ�������FeԪ�ص�ƽ�����ϼ�Ϊx�����ݵ�ʧ�����غ㣬0.3mol![]() [x-(+2)]=0.1mol
[x-(+2)]=0.1mol![]() [0-(-2)]�����x=
[0-(-2)]�����x=![]() �������������ϼ۴�����Ϊ0���Ļ�ѧʽΪFe3S4��
�������������ϼ۴�����Ϊ0���Ļ�ѧʽΪFe3S4��
��1��������������֪���Ļ�ѧʽΪFe3S4���ʼ���Fe��S����Ԫ����ɣ�
�������Fe��S��
��2����������Ũ���ᷴӦ�����ӷ���ʽΪ��Fe3S4 +6H+=3Fe2++S��+H2S����
�������Fe3S4 +6H+=3Fe2++S��+H2S����
��3����������H2S���ڹ��������г��ȼ�պ�����SO2����ȼ�պ�Ļ�����壨SO2��O2��ͨ��BaCl2��Һ�����ְ�ɫ����BaSO4�����ӷ���ʽΪ��2SO2+O2+2H2O+2Ba2+=2BaSO4�� +4H+��
�������2SO2+O2+2H2O+2Ba2+=2BaSO4 ��+4H+��

����Ŀ����NaClΪ��Ҫ�ɷֵ���ѩ���ḯʴ����������ȸ����豸��ij�о�С��̽��NaCl��Һ�Ը�����ʴ��Ӱ�졣
��1������ֽ��3.5%��NaCl��Һ��ʪ��Ϳ�����ۡ�̼�۵Ļ������ڱ������ϡ�����ֽ�ϼӼ��μ����Լ����ٻ�������NaCl��Һ��û����ֽ������������ʾ��

��ʵ����������˵�����õ��ӵ�������_______________________________��
��̼�۵�������___________________________________________________��
��Ϊ��˵��NaCl�����ã���Ҫ����Ķ���ʵ����_____________________��
��2������ͼʾװ�õ��ձ�a��b�и�����30mL 3.5%��NaCl��Һ���պ�K��ָ��δ����ƫת�������ձ�a��ָ������ƫת��

��ȡa��b����Һ�������μ�K3[Fe(CN)6]��Һ��a�г�����ɫ������b���ޱ仯��b����Ƭ��________����
�ڼ��Ⱥ�ָ�뷢��ƫת��ԭ�������_____________________��
��3���ã�2����ͼʾװ��̽����ͬŨ��NaCl��Һ�Ը�����ʴ��Ӱ�죬���ձ�a��b�и�����30mL��ͬ����������NaCl��Һ��ʵ���¼���±���ʾ��
ʵ�� | a | b | ָ��ƫת���� |
I | 0.1% | 0.01% | ���� |
II | 0.1% | 3.5% | ���� |
�� | 3.5% | ������Һ | ���� |
�����У�b�е缫�����ĵ缫��Ӧʽ��_______________________________��
�����У����ڱ���NaCl��Һ�в��ױ���ʴ���������Ͽ�֪���ڱ���NaCl��Һ��O2Ũ�Ƚϵͣ��������ױ���ʴ�����ʵ��֤����_______________________________��
��4����������ʵ�飬�Ը�����ʴ��Ӱ���������_______________________________��