��Ŀ����
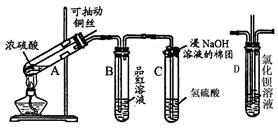
��12�֣������dzµ��㡱��������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ��������ͼ��ʾ��װ����ȡ�����������ش��������⣺
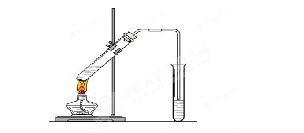
(1)д����ȡ���������Ļ�ѧ��Ӧ����ʽ ��
(2)����̼������Һ����Ҫ������ ��
(3)Ũ����������� �� ��
(4)װ����ͨ�����ĵ���Ҫ���ڱ���̼������Һ��Һ���ϣ����ܲ�����Һ�У�Ŀ���Ƿ�ֹ ��
(5)��Ҫ���Ƶõ������������������Ӧ���õ�ʵ����������� ��

(1)д����ȡ���������Ļ�ѧ��Ӧ����ʽ ��
(2)����̼������Һ����Ҫ������ ��
(3)Ũ����������� �� ��
(4)װ����ͨ�����ĵ���Ҫ���ڱ���̼������Һ��Һ���ϣ����ܲ�����Һ�У�Ŀ���Ƿ�ֹ ��
(5)��Ҫ���Ƶõ������������������Ӧ���õ�ʵ����������� ��
��1��CH3COOH + C2H5OH 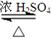 CH3COOC2H5 + H2O
CH3COOC2H5 + H2O
(2)��ȴ���������е��ܽ�ȸ�С�����������룬��ȥ�ֲ�Ʒ�е������������ŵ�����ζ
(3)���� (4)���� (5)��Һ
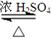 CH3COOC2H5 + H2O
CH3COOC2H5 + H2O(2)��ȴ���������е��ܽ�ȸ�С�����������룬��ȥ�ֲ�Ʒ�е������������ŵ�����ζ
(3)���� (4)���� (5)��Һ
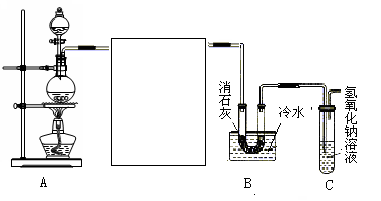
��1��ʵ������������Ҵ���Ũ�����������ͨ��������Ӧ��������������
��2�����ڲ��������������к��лӷ�����������Ҵ������ñ���̼������Һ��ȥ�����������������е��ܽ�ȸ�С�����������룬��ȥ�ֲ�Ʒ�е�������Ҵ��������ŵ�����ζ��
��3����Ϊ������Ӧ�ǿ���ģ�����Ũ�����������������⣬������ˮ�������á�
��4�������Ҵ������ᶼ�Ǻ�ˮ���ܵģ����Բ��ܲ�����Һ�е�Ŀ���Ƿ�ֹ������
��5����������������ˮ����Һ����ʵ�ַ��롣
��2�����ڲ��������������к��лӷ�����������Ҵ������ñ���̼������Һ��ȥ�����������������е��ܽ�ȸ�С�����������룬��ȥ�ֲ�Ʒ�е�������Ҵ��������ŵ�����ζ��
��3����Ϊ������Ӧ�ǿ���ģ�����Ũ�����������������⣬������ˮ�������á�
��4�������Ҵ������ᶼ�Ǻ�ˮ���ܵģ����Բ��ܲ�����Һ�е�Ŀ���Ƿ�ֹ������
��5����������������ˮ����Һ����ʵ�ַ��롣

��ϰ��ϵ�д�
 һ����ʦ�����Ծ�ϵ�д�
һ����ʦ�����Ծ�ϵ�д�
�����Ŀ