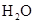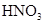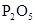��Ŀ����
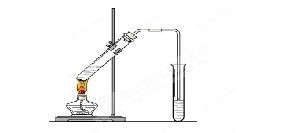
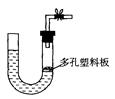
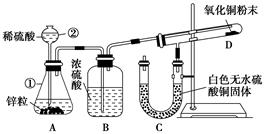
��16�֣�ijѧ����������װ��̽�������백��֮��ķ�Ӧ������A��F�ֱ�Ϊ�����������ķ���װ�ã�CΪ��������������백����Ӧ��װ�á�

��ش��������⣺
(1)װ��F�з�����Ӧ�����ӷ���ʽΪ ��
(2)װ��A����ƿ�п�װ�Լ� ��
(3)Bװ�õ������� ��Eװ�õ����� ��
(4)ͨ��Cװ�õ�����������߽ϳ����ұ߽϶̣�Ŀ���� ��
(5)װ��C�ڳ���Ũ��İ��̲��������ڱ����ᣬ��һ�������ǿ�������Ҫ�ɷ�֮һ����д����Ӧ�Ļ�ѧ����ʽ�� ��
����a mol�����μӷ�Ӧʱ��ת�Ƶĵ�������Ϊb��������ӵ���������Ϊ(�ú�a��b�Ĵ���ʽ��ʾ)
(6)��װ��C��G���ݳ���β���п��ܺ��л���ɫ���ж����壬��δ���?
��

��ش��������⣺
(1)װ��F�з�����Ӧ�����ӷ���ʽΪ ��
(2)װ��A����ƿ�п�װ�Լ� ��
(3)Bװ�õ������� ��Eװ�õ����� ��
(4)ͨ��Cװ�õ�����������߽ϳ����ұ߽϶̣�Ŀ���� ��
(5)װ��C�ڳ���Ũ��İ��̲��������ڱ����ᣬ��һ�������ǿ�������Ҫ�ɷ�֮һ����д����Ӧ�Ļ�ѧ����ʽ�� ��
����a mol�����μӷ�Ӧʱ��ת�Ƶĵ�������Ϊb��������ӵ���������Ϊ(�ú�a��b�Ĵ���ʽ��ʾ)
(6)��װ��C��G���ݳ���β���п��ܺ��л���ɫ���ж����壬��δ���?
��
(1)MnO2+4H++2Cl-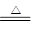 Mn2++Cl2��+2H2O(2��)
Mn2++Cl2��+2H2O(2��)
(2)��ʯ�һ�����������ƻ��ʯ��(2��)
(3)����ܣ�2�֣� ��ȥ�����е��Ȼ��⣨2�֣�
(4)ʹ�ܶȴ���������ܶ�С�İ����Ͽ�ؾ��Ȼ��(2��)
(5)3C12+8NH3��N2+6NH4Cl(2��) b/2a mol-1(2��)
(6)��G���ӵ���ֱ��ͨ��ʢ���ռ���ձ��У�2�֣�
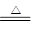 Mn2++Cl2��+2H2O(2��)
Mn2++Cl2��+2H2O(2��)(2)��ʯ�һ�����������ƻ��ʯ��(2��)
(3)����ܣ�2�֣� ��ȥ�����е��Ȼ��⣨2�֣�
(4)ʹ�ܶȴ���������ܶ�С�İ����Ͽ�ؾ��Ȼ��(2��)
(5)3C12+8NH3��N2+6NH4Cl(2��) b/2a mol-1(2��)
(6)��G���ӵ���ֱ��ͨ��ʢ���ռ���ձ��У�2�֣�
��1��F����ȡ�����ģ�����ʽΪMnO2+4H++2Cl-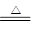 Mn2++Cl2��+2H2O��
Mn2++Cl2��+2H2O��
��2��װ��A����ȡ�����ģ����ڰ�ˮ�д���ƽ��NH3��H2O NH3��H2O
NH3��H2O NH4����OH��������Ҫ���ɰ�����Ӧ��ʹƽ�����淴Ӧ�����ƶ�����˿�������ʯ�һ�����������ƻ��ʯ�ҵȡ�
NH4����OH��������Ҫ���ɰ�����Ӧ��ʹƽ�����淴Ӧ�����ƶ�����˿�������ʯ�һ�����������ƻ��ʯ�ҵȡ�
��3������װ�õĽṹ��֪B�Ǹ���ܡ�Ũ�����ӷ����������ɵ������к����Ȼ������壬��˱���ʳ��ˮ�������dz�ȥ�����е��Ȼ��⡣
��4���������ܶȴ��ڿ����ģ��������ܶ�С�ڿ����ģ�����Cװ�õ�����������߽ϳ����ұ߽϶̵�Ŀ�ľ���ʹ�ܶȴ���������ܶ�С�İ����Ͽ�ؾ��Ȼ�ϡ�
��5��C�ڳ���Ũ��İ��̲��������ڱ����ᣬ˵�����Ȼ�����ɡ���һ�������ǿ�������Ҫ�ɷ�֮һ�����ǵ��������Է���ʽΪ3C12+8NH3��N2+6NH4Cl�����ݷ���ʽ��֪a mol������Ӧת��2amol���ӣ����Է��ӵ���������Ϊ b/2a mol-1��
��6�������ж�����Ҫβ������װ�ã����Է�������G���ӵ���ֱ��ͨ��ʢ���ռ���ձ��С�
 Mn2++Cl2��+2H2O��
Mn2++Cl2��+2H2O����2��װ��A����ȡ�����ģ����ڰ�ˮ�д���ƽ��NH3��H2O
 NH3��H2O
NH3��H2O NH4����OH��������Ҫ���ɰ�����Ӧ��ʹƽ�����淴Ӧ�����ƶ�����˿�������ʯ�һ�����������ƻ��ʯ�ҵȡ�
NH4����OH��������Ҫ���ɰ�����Ӧ��ʹƽ�����淴Ӧ�����ƶ�����˿�������ʯ�һ�����������ƻ��ʯ�ҵȡ���3������װ�õĽṹ��֪B�Ǹ���ܡ�Ũ�����ӷ����������ɵ������к����Ȼ������壬��˱���ʳ��ˮ�������dz�ȥ�����е��Ȼ��⡣
��4���������ܶȴ��ڿ����ģ��������ܶ�С�ڿ����ģ�����Cװ�õ�����������߽ϳ����ұ߽϶̵�Ŀ�ľ���ʹ�ܶȴ���������ܶ�С�İ����Ͽ�ؾ��Ȼ�ϡ�
��5��C�ڳ���Ũ��İ��̲��������ڱ����ᣬ˵�����Ȼ�����ɡ���һ�������ǿ�������Ҫ�ɷ�֮һ�����ǵ��������Է���ʽΪ3C12+8NH3��N2+6NH4Cl�����ݷ���ʽ��֪a mol������Ӧת��2amol���ӣ����Է��ӵ���������Ϊ b/2a mol-1��
��6�������ж�����Ҫβ������װ�ã����Է�������G���ӵ���ֱ��ͨ��ʢ���ռ���ձ��С�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

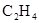
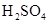
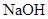 ��Һ
��Һ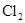
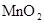
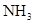
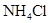 ��Һ
��Һ