��Ŀ����
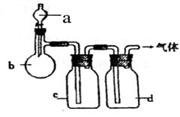
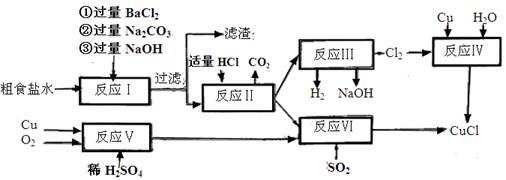
��12�֣�����������Ԫ����ص�һϵ��ʵ�飺������ͼ��ʾʵ��ش��������⣺
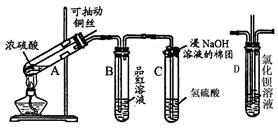
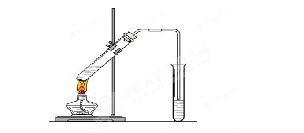
��1��A�з����ķ�Ӧ����ʽ�� ��
��2���Թ�C�ڲ��������Ϸ�����Ӧ�����ӷ���ʽ�� ��
��3����Ӧһ��ʱ���ֹͣ��Ӧ����B�Թ��е���Һ���ȣ����Թ۲쵽 ���Թ�C����Һ����ǣ���֤��SO2���� �ԡ�
��4���罫B�Թܻ���D�Թܣ�����ֱ����������BaCl2��Һ��ͨ��Cl2���壬����
��5�������Ӧ������Ҫ����ֹͣ��Ӧ����ķ����� ��

��1��A�з����ķ�Ӧ����ʽ�� ��
��2���Թ�C�ڲ��������Ϸ�����Ӧ�����ӷ���ʽ�� ��
��3����Ӧһ��ʱ���ֹͣ��Ӧ����B�Թ��е���Һ���ȣ����Թ۲쵽 ���Թ�C����Һ����ǣ���֤��SO2���� �ԡ�
��4���罫B�Թܻ���D�Թܣ�����ֱ����������BaCl2��Һ��ͨ��Cl2���壬����
��5�������Ӧ������Ҫ����ֹͣ��Ӧ����ķ����� ��
��1��Cu+2H2SO4==CuSO4+SO2��+2H2O ��2��SO2+2OH- == SO32-+2H2O
��3����ɫ���ɫ�������ԡ� ��4����ɫ���� ��5�����ͭ˿��Һ���ϡ�
��3����ɫ���ɫ�������ԡ� ��4����ɫ���� ��5�����ͭ˿��Һ���ϡ�
�����������1��A�з����ķ�Ӧ��Cu+2H2SO4==CuSO4+SO2��+2H2O
��2��C�ڲ���������Ҫ�����ն����SO2����Ӧ�����ӷ���ʽΪ��SO2+2OH- == SO32-+2H2O
��3��B���е�Ʒ����Ϊͨ��SO2����ɫ�����Ⱥ��ɫ�ָ���C���е�H2S��SO2��Ӧ�������ʣ�˵��SO2���������ԡ�
��4��D���з����ķ�Ӧ��Cl2+SO2+2H2O=2HCl+H2SO4��H2SO4+BaCl2=BaSO4��+2HCl�����Կ��Կ����а�ɫ�������ɡ�
��5�����������ֹͣ��Ӧ����ͭ˿������ɡ�
������������Ҫ����ѧ���۲�������������ͽ�������������

��ϰ��ϵ�д�
�����Ŀ
 ��
��