��Ŀ����
�����̿�MnCO3��������Fe2O3��FeO��HgCO3��2HgO�����ʣ���ҵ�������̿���ȡ�̣������������£�
��ش��������⣺
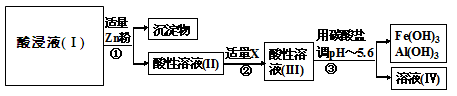
��1�����Һ1�м����ˮ�����Ҫ �������ܴﵽ����Ҫ��
��2���������õĿ�������Ĥ���뷨�Ʊ��ĸ����������÷�����ԭ���� ��
��3����������Ҫ�ɷ�Ϊ(NH4)2S����Һ2�з�����Ҫ��Ӧ�����ӷ���ʽΪ ��
��4��д�������ĵ缫��Ӧʽ ��˵�����Һѭ����ԭ�� ��
��5��д�����ȷ����̵Ļ�ѧ����ʽ ��
��1������ ��2�������е������͵���������Ĥ��������ͬ
��3��Hg2++S2?= HgS��
��4��Mn2++2H2O��2e?=MnO2+4H+������δ��Ӧ��Mn2+����߶������̵IJ��ʡ�
��5��3MnO2+4Al 2Al2O3+3Mn (2�� )
2Al2O3+3Mn (2�� )
����������������������Ϣ֪�����̿����Ҫ�ɷ���̼���̣�����Fe2O3��FeO��HgCO3?2HgO�����ʣ������̿�����ᣬ�������ṯ�������̡���������������������Һ�ȣ���������������Ϊ�����ӣ�ͨ������pHʹ������ת��Ϊ����������������ȥ���������ɽ��ؽ�������ת��Ϊ��������Һ��Ҫ�������̣����ʱ�����ӷŵ����ɶ������̣�Ȼ���������ȷ�Ӧ�Ʊ�Mn����1�����Һ1�м����ˮ�����Ҫ����ﵽŨ����Ŀ�ģ�ʹ���ʵ�Ũ������2���������õĿ�������Ĥ���뷨�Ʊ��ĸ�����������ԭ��Ϊ�����е������͵���������Ĥ��������ͬ����3����Һ2�мӾ�����(NH4)2S������Ҫ��Ӧ�����ӷ���ʽΪHg2++S2-=HgS������4����Һ��Ҫ�������̣����ʱ�����ӷŵ����ɶ������̣���������ӦʽΪMn2++2H2O-2e-=MnO2+4H+�����Һѭ�������������δ��Ӧ��Mn2+����߶������̵IJ��ʣ���5�����ȷ����̵Ļ�ѧ����ʽΪ3MnO2+4Al 2Al2O3+3Mn��
2Al2O3+3Mn��
���㣺���黯ѧ�뼼����

 ������ȫ�̼����ĩ���100��ϵ�д�
������ȫ�̼����ĩ���100��ϵ�д���ҵ�ϳ�����ұ��п�����е�п(����ZnO��FeO��Fe2O3��CuO��Al2O3������)������������ZnSO4��6H2O���壬�乤���������£��й������������ʱ��pH���±���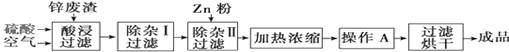
| �������� | Al(OH)3 | Fe(OH)3 | Fe(OH)2 | Zn(OH)2 |
| ��ʼ������pH | 3.3 | 1.5 | 6.5 | 5.4 |
| ������ȫ��pH | 5.2 | 3.7 | 9.7 | 8.0 |
��1���������������жദ�漰�����ˡ���ʵ�����й��˲�����Ҫʹ�õIJ��������� ��
��2���ڡ����Ӣ����У����ټ�������H2O2��Һ��Ŀ���� ��
ΪʹFe(OH)3��Al(OH)3������ȫ����Zn(OH)2��������Ӧ������Һ��pH��ΧΪ ��
Ϊ��������PH��Χ��ѡ�������Լ���ҩƷ�� ��
A��ZnO B����ˮ C������NaOH D��ZnCO3
��3���ڡ����Ӣ����У�����Zn�۵������� ��������A���������� ��
��4�������£���֪Ksp��Cu(OH)2����2��10��20��ijCuSO4��Һ��c��Cu2������0.02 mol��L��1�����Ҫ����Cu(OH)2��������Ӧ������ҺpH���� ��
��ͭ����Ҫ��xCuCO3��yCu(OH)2������������Fe�Ļ������ҵ������ͭ��Ϊԭ���Ʊ�Cu��CaCO3��CuSO4��5H2O�����巽���������£�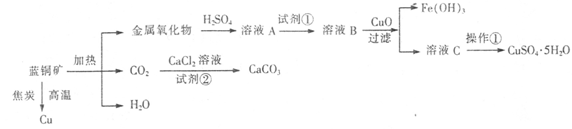
��֪��
| �������� | Fe3+ | Fe2+ | Cu2+ | |
| pH | �������↑ʼ���� | 1.9 | 7.0 | 4.7 |
| ����������ȫ���� | 3.2 | 9.0 | 6.7 | |
��1����ͭ�����Ҫ�ɷ��뽹̿����������������ͭ��������̼��ˮ��д���÷�Ӧ�Ļ�ѧ����ʽ
��
��2�������������Լ���ѡ��ʵ�鲽�����Լ���Ϊ ������ţ���
a��KMnO4 b��K2Cr2O7 c��H2O2 d����ˮ
��3������ҺB�м���CuO�������ǵ�����ҺpH����pH�ķ�ΧΪ ��
��4������ҺC���CuSO4��5H2O����Ҫ������������Ũ������ȴ�ᾧ�����˵Ȳ��������������� ʱֹͣ���ȡ�
��5���Ʊ�CaCO3ʱ��Ӧ��CaCl2��Һ����ͨ��(����룩�Լ��ڣ����Լ��ڿ�����
(����ţ���
a����ˮ b������ c��ˮ���� d��NaOH��Һ
����������Լ��ڣ���CaCl2��Һ������CO2��Ӧ����CaCO3�����������ܵ���ʵij����ܽ�ƽ��ԭ���������ܵ�ԭ�� ��
��6��Ϊȷ���Լ��ٵ���������ⶨ��ҺA��Fe2+��Ũ�ȡ�ʵ�����Ϊ��ȷ��ȡ20.00mL ��ҺA����ƿ�У���0.01200 mol/L������KMnO4����Һ�ζ����յ㣬����KMnO4����Һ15.00 mL������ҺA��Fe2+��Ũ��Ϊ ��
ﮱ���Ϊ������ζ��������LiCoO2Ϊ�������ϵ�����ӵ���ѱ��㷺������Яʽ��Դ����ҵ�ϳ��Ԧ�﮻Կ�(��Ҫ�ɷ�ΪLiAlSi2O6��������FeO��MgO��CaO������)Ϊԭ������ȡ����ﮡ�����һ�ֹ����������£�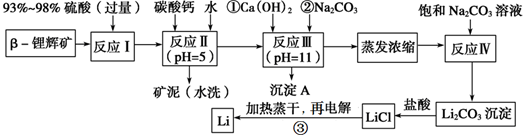
��֪�����ֽ����������↑ʼ��������ȫ����ʱ��pH��
| �������� | Fe(OH)3 | Al(OH)3 | Mg(OH)2 |
| ��ʼ����pH | 2.7 | 3.7 | 9.6 |
| ��ȫ����pH | 3.7 | 4.7 | 11 |
��ش��������⣺
��1��д����Ӧ�����е���ת�ƵĻ�ѧ����ʽ_________________________��
��2����Ӧ�����̼��Ƶ�������_______________��
��3����ͬѧ��Ϊ�ڷ�Ӧ����ֻ�����̼������ҺҲ�ܴ�ɸò����Ŀ�ģ��������Ĺ۵������ ____________��
��4��������������Ũ�����ڣ�����ʵ���ҽ��иò�����������Ũ����________����ʱ���Ϳ�ֹͣ�ò�����
��5��Li��Mg��Be��Al��B��Si������Ԫ�������ڱ��д��ڶԽ���λ�ã���Ӧ����Ԫ�ؼ��仯�������������������֮�������������Գ�Ϊ�Խ��߹�������е��ʱ�����������������л��������������ԭ����___________��
��6�������һ�ִ�ˮϴ��Ŀ����з����Al2O3�����̣�
�����̳��õı�ʾ��ʽΪ��
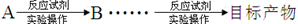 ��
�� ����þ��ҽҩ����������ҵӦ�ù㷺������þ��ԭ�Ƚ��Ʊ��ߴ�����þ��һ���µ�̽��������þ��(��Ҫ�ɷ�ΪMgCO3��������FeCO3 )Ϊԭ���Ʊ��ߴ�����þ��ʵ���������£�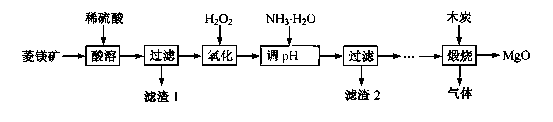
�����������������pH
| | Mg(OH)2 | Fe(OH)2 | Fe(OH)3 |
| ��ʼ����ʱ | 9.4 | 6.3 | 1.5 |
| ��ȫ����ʱ | 12.4 | 8.3 | 2.8 |
��1��MgCO3��ϡ���ᷴӦ�����ӷ���ʽΪ ��
��2���Ӱ�ˮ������Һ��PH��ΧΪ ��
��3������2 �ijɷ��� (�ѧʽ)��
��4�����չ��̴������·�Ӧ��
2MgSO4+C
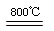 2MgO+2SO2��+CO2��
2MgO+2SO2��+CO2��MgSO4+C
 MgO+SO2��+CO��
MgO+SO2��+CO�� MgSO4+3C
 MgO+S��+3CO��
MgO+S��+3CO��������ͼװ�ö����ղ�����������зֲ����ջ��ռ���

��D���ռ��������� (�ѧʽ)��
��B��ʢ�ŵ���Һ�� (����ĸ)��
a��NaOH ��Һ b��Na2CO3��Һ c��ϡ���� d��KMnO4��Һ
��A�еõ��ĵ���ɫ�������ȵ�NaOH��Һ��Ӧ��������Ԫ�����̬Ϊ+4��д���÷�Ӧ�����ӷ���ʽ�� ��