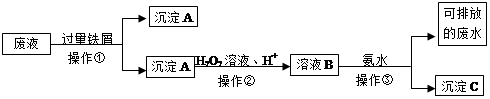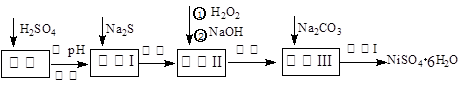��Ŀ����
�ߴ���ϸ�������������ںϳ�����﮵�ص缫���ϣ���ҵ�Ͽ�������ȡ�Ѱ۵ĸ���Ʒ�̷���FeSO4��7H2O��ͨ�����з�Ӧ�Ʊ���
FeSO4��2NH3��H2O��Fe(OH)2����(NH4)2SO4
Fe(OH)2��H2C2O4��FeC2O4��2H2O
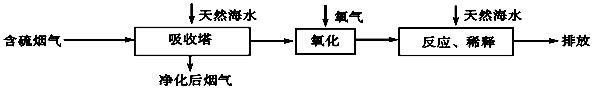
��1���̷��к���һ������TiOSO4���ʡ����̷�����ϡ���ᣬ�������ۡ����衢��ַ�Ӧ������һ��ʱ�䣬���ˣ��ɵô�����FeSO4��Һ�������������У�TiOSO4����ˮ��Ӧת��ΪH2TiO3������д���÷�Ӧ�Ļ�ѧ����ʽ�� ���������۵������� �� ��
��2���ɴ�����FeSO4��Һ��ȡFeC2O4ʱ��������ջ����½��У�ԭ���� ��
FeC2O4���ɺ�Ϊ��߲�Ʒ���ȣ����������ҺpH��2����pH���ͣ�����
FeC2O4�IJ��� ���ƫ�ߡ�����ƫ�͡�����Ӱ�족����
��3��������FeC2O4�Ļ��Һ���ˣ�����Ʒ����ˮϴ�ӣ�������ˮ�Ҵ���ϴ����ˮ�Ҵ��������� �� ��
��4��ij�о�С������ij������������Ҫ�ɷ�ΪFe2O3��SiO2��Al2O3�����������Ʊ��ϴ�����FeSO4��Һ���ٺϳ�FeC2O4���벹�������ɸû��������Ʊ��ϴ�����FeSO4��Һ��ʵ�鲽�裨��ѡ�õ��Լ������ۡ�ϡ�����NaOH��Һ������һ�����û��������м���������ϡ�����ַ�Ӧ�����ˣ� �����ˣ��õ��ϴ�����FeSO4��Һ��
��1��TiOSO4��2H2O��H2TiO3����H2SO4��2�֣�
��ֹFe2+��������������ҺpH����ȥ���ʣ������𰸾��ɣ�
��2���������ɵ�Fe(OH)2�����ʱ������������𰸾��ɣ���2�֣� ƫ�ͣ�2�֣�
��3����ȥ�����������ʡ�ʹ��Ʒ���ٸ�����ٲ�Ʒ����ĵȣ�2�֣�ÿ���1���1�֣�
��4������Һ�еμӹ�����NaOH��Һ�����ˣ����ϴ�ӹ��壬������м�������ϡ������������ȫ�ܽ⣬�ټ������������ۣ���ֽ����3�֣�
���������������1����Һ�е����������ױ������е��������������Լ������۷�ֹFe2+����������������Һ�������ӷ�Ӧ���ٽ�TiO2+ˮ����ȫ�����Ӷ���ȥ����2�����Ʊ������з�Ӧ���ɸ��ױ�������Fe(OH)2���������������Ƿ�ֹ������������ΪFe(OH)3��ʵ�����������Һ���Թ�ǿ��������ʽ�������������²���ƫ�͡���3���Ҵ����н�ǿ�ӷ��ԣ�������ˮϴ�������Ҵ�ϴ����Ȼ��ʹ��Ʒ���ٸ����ֹ�ڼ��Ȼ��ڿ����л����������Ӧ�Բ�Ʒ����������Ӱ�죻��4���Ʊ�������FeSO4��Һ��Ҫ��ȥ���ʣ��ȼ����ܽ���˳�ȥ�������裬�ټӹ������ȥ�����ӣ��ټ����ᡢ���۵ȵõ�������FeSO4��Һ��
���㣺�����ۺ�ʵ���й��̵Ŀ��ơ�ԭ���������й����⡣

 ������ÿ�ʱ�Ż���ҵϵ�д�
������ÿ�ʱ�Ż���ҵϵ�д�
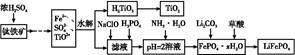
 ������ǿ�����ԣ�������ԭΪ����أ���80 �����������ֽ⡣ʵ����ģ�ҵ�ϳɹ�����ص��������£�
������ǿ�����ԣ�������ԭΪ����أ���80 �����������ֽ⡣ʵ����ģ�ҵ�ϳɹ�����ص��������£�



 KCl+KClO+H2O(����:�¶Ƚϵ�)
KCl+KClO+H2O(����:�¶Ƚϵ�) ;��������������������
;��������������������