��Ŀ����
��ͭ����Ҫ��xCuCO3��yCu(OH)2������������Fe�Ļ������ҵ������ͭ��Ϊԭ���Ʊ�Cu��CaCO3��CuSO4��5H2O�����巽���������£�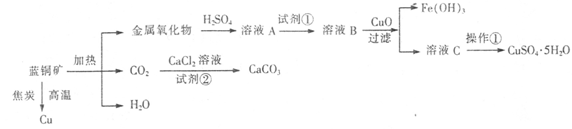
��֪��
| �������� | Fe3+ | Fe2+ | Cu2+ | |
| pH | �������↑ʼ���� | 1.9 | 7.0 | 4.7 |
| ����������ȫ���� | 3.2 | 9.0 | 6.7 | |
��1����ͭ�����Ҫ�ɷ��뽹̿����������������ͭ��������̼��ˮ��д���÷�Ӧ�Ļ�ѧ����ʽ
��
��2�������������Լ���ѡ��ʵ�鲽�����Լ���Ϊ ������ţ���
a��KMnO4 b��K2Cr2O7 c��H2O2 d����ˮ
��3������ҺB�м���CuO�������ǵ�����ҺpH����pH�ķ�ΧΪ ��
��4������ҺC���CuSO4��5H2O����Ҫ������������Ũ������ȴ�ᾧ�����˵Ȳ��������������� ʱֹͣ���ȡ�
��5���Ʊ�CaCO3ʱ��Ӧ��CaCl2��Һ����ͨ��(����룩�Լ��ڣ����Լ��ڿ�����
(����ţ���
a����ˮ b������ c��ˮ���� d��NaOH��Һ
����������Լ��ڣ���CaCl2��Һ������CO2��Ӧ����CaCO3�����������ܵ���ʵij����ܽ�ƽ��ԭ���������ܵ�ԭ�� ��
��6��Ϊȷ���Լ��ٵ���������ⶨ��ҺA��Fe2+��Ũ�ȡ�ʵ�����Ϊ��ȷ��ȡ20.00mL ��ҺA����ƿ�У���0.01200 mol/L������KMnO4����Һ�ζ����յ㣬����KMnO4����Һ15.00 mL������ҺA��Fe2+��Ũ��Ϊ ��
��1��2[xCuCO3��yCu(OH)2] + (x+y)C ="==" 2(x+y)Cu + (3x+y)CO2 + 2yH2O ( 2��)
��2��c (2��)
��3��3.2��pH<4.7 (2��)
��4����������������(���߱�����ֽᾧ��Ĥ) (2��)
��5�� ad ��2�֣� H2CO3�ڶ������������CO32-����Ũ��̫�ͣ���Ca2+Ũ�ȵij˻�С��CaCO3���ܶȻ��������������CaCO3����������������ʺ�CO32-����Ũ��������Ca2+Ũ�ȵij˻�������CaCO3���ܶȻ������������CaCO3���������������ɣ�2��)
��6�� 0.04500mol/L (2�֣���Ч���ִ����1�֣�
���������������˫��ˮ��һ����ɫ�������������������ʣ����ѡC���Ǽ���CuO�ǵ�����Һ��pH��Ŀ���dz�ȥ��Һ�е����������ӣ���������ͭ���ӳ������������Ӧ������3.2��pH<4.7����Ҫ�������Ƚ�ʣ���ˮ�����ɡ�����Ҫʹ��Һ�Լ��ԣ��Ȼ�����Һ��ͨ�������̼��������̼��Ƴ�����
�� KMnO4��������������������5Fe2+
1mol 5mol
0.01200 mol/L��15.00 mL c(Fe2+)��20.00mL
��֮�ã�c(Fe2+)��0.04500mol/L
���㣺����������ԭ��Ӧ����ʽ����д������ʱ��Һ����Ե�ѡ��ȡ�

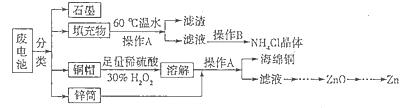
�����к��ḻ�ĵ�Ԫ�أ��Ӻ����з������ɰ����²�����У��ٵμ�ϡ�����ữ��������H2O2����������Ϊ3%�� �ڽ��������ճɻҺ��ˮ���� �ۼ�CCl4�� ���÷�Һ©����Һ ����С����ˡ������IJ���˳����
| A���٢ڢۢܢ� | B���٢ۢݢڢ� | C���ڢݢ٢ۢ� | D���ڢ٢ۢݢ� |
ij�о���ѧϰС���������ռ���������Ϣ���ء��ơ��ơ�þ�Ȼ��ý���������CO2������ȼ�ա����Ƕ�����CO2������ȼ�պ�IJ����еİ�ɫ���ʽ���������̽����
��ʵ�顿��ȼ�յ���Ѹ�����뵽ʢ��CO2�ļ���ƿ�У��������м���ȼ�գ���Ӧ����ȴ��ƿ���ź�ɫ������ƿ����𤸽�Ű�ɫ���ʡ�
��������衿
����1����ɫ������Na2O��
����2����ɫ������Na2CO3��
����3����ɫ������Na2O��Na2CO3�Ļ���
�����ʵ�鷽����֤���衿 ��С���ȼ�պ�İ�ɫ����
��������̽����
| ʵ�鷽�� | ʵ����� | ʵ������ | ���� |
| ����1 | ȡ������ɫ�������Թ��У���������ˮ������Ʒȫ������ˮ�������м�����ɫ��̪��Һ | ��Һ��ɺ�ɫ | ��ɫ����ΪNa2O |
| ����2 | ��ȡ������ɫ�������Թ��У���������ˮ������Ʒȫ������ˮ�������м��������CaCl2��Һ | ���ְ�ɫ���� | |
| | �ھ���Ƭ�̣�ȡ�ϲ���Һ���Թ��У��μ���ɫ��̪��Һ | ���������� |
��˼���뽻����
(1)��ͬѧ��Ϊ����1�õ��Ľ��۲���ȷ���������� ��
(2)��ͬѧ��Ϊ����2�õ��Ľ�����ȷ����ɫ����Ϊ ��
(3)ͨ������1�ͷ���2��ʵ�飬����Ϊ�������������У� ��������������� ��
(4)���ڶ�����̼��ȼ�յĻ�ѧ����ʽΪ ��
(5)��ͬѧ��Ϊ��ɫ�����п������������ơ����Ƿ�ͬ���ͬѧ�Ĺ۵㣿���������ɣ� ��



 ��Һ�ζ����յ㣬����
��Һ�ζ����յ㣬���� ��Һ�����ƽ��ֵΪ19��00mL��
��Һ�����ƽ��ֵΪ19��00mL��

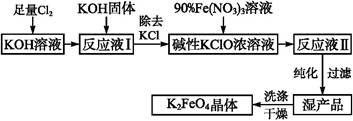
 KCl+KClO+H2O(����:�¶Ƚϵ�)
KCl+KClO+H2O(����:�¶Ƚϵ�) ;��������������������
;��������������������