��Ŀ����
���ù�������KxFe��C2O4��y��3H2O��FeΪ��3�ۣ���ʵ�����Ʊ��Ͳⶨ����ɵķ���������ʾ��
��.�Ʊ���
��1����K2C2O4��FeCl3�Ʊ��������ϵķ�Ӧ����________������ţ���
�����ӷ�Ӧ���ڷ�������ԭ��Ӧ����������ԭ��Ӧ���ܻ��Ϸ�Ӧ
��2���ᾧʱӦ��������Һ���ںڰ����ȴ����������������������ԭ����_________��
��3������4��ʵ�������____________��
��.��ɲⶨ��
��ȡһ������ʵ�����õľ���������ƿ�У�����������ˮ��ϡH2SO4����C2O42-��ȫת��ΪH2C2O4����0.10 mol��L��1 KMnO4��Һ���еζ�������KMnO4��Һ24.00 mLʱǡ����ȫ��Ӧ������������MnO4-�Ļ�ԭ������Mn2�������ټ��������Ļ�ԭ������Fe3����ȫת��ΪFe2������KMnO4��Һ�����ζ�����Fe2����ȫ����ʱ����ȥKMnO4��Һ4.00 mL��
��4�������������H2C2O4��Fe2�������ӷ���ʽ�ֱ���___________�� ________��
��5������100 mL 0.10 mol��L��1 KMnO4��Һ���������ζ�ʵ��������IJ����������ձ�������������ͷ�ιܡ���Ͳ����ƿ�⣬����________��________��д���ƣ���
��6��ͨ�����㣬�û�����Ļ�ѧʽ��____________��
��.��1���٢�
��2����ֹ�������ֽ�
��3�����ˡ�ϴ�ӡ�����
��.��4��2MnO42-��5H2C2O4��6H��=2Mn2����10CO2����8H2O��MnO4-��5Fe2����8H��=Mn2����5Fe3����4H2O
��5��100 mL����ƿ����ʽ�ζ���
��6��K3Fe��C2O4��3��3H2O
����

��һ��ɫ����Һ����ȷ���Ƿ����������ӣ� Fe2����Mg2����Al3����Ba2���� ��
�� ��Cl����I����
��Cl����I���� ��ȡ����Һ����ʵ�飺
��ȡ����Һ����ʵ�飺
| ʵ�鲽�� | ʵ������ |
| (1)ȡ��������Һ���Ӽ�����ɫʯ����Һ | ��Һ��� |
| (2)ȡ��������Һ���ȣ���CuƬ��ŨH2SO4������ | ����ɫ���������������������ɺ���ɫ |
| (3)ȡ��������Һ����BaCl2��Һ | �а�ɫ���� |
| (4)ȡ(3)���ϲ���Һ����AgNO3��Һ | �а�ɫ�������Ҳ�����ϡHNO3 |
| (5)ȡ��������Һ����NaOH��Һ | �а�ɫ������NaOH����ʱ���������ܽ� |
�ɴ��жϣ�
(1)��Һ�п϶������ڵ������� ����Һ�п϶����ڵ������� ��
(2)�����ʵ����֤���п��ܴ��ڵ������ӵķ���(д��������������) ��

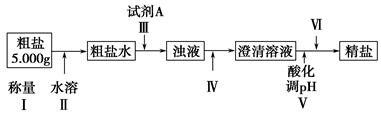


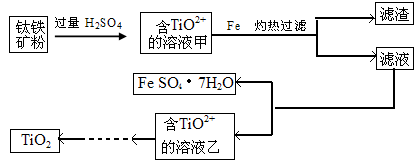
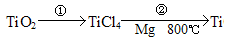
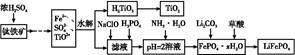
 ������ǿ�����ԣ�������ԭΪ����أ���80 �����������ֽ⡣ʵ����ģ�ҵ�ϳɹ�����ص��������£�
������ǿ�����ԣ�������ԭΪ����أ���80 �����������ֽ⡣ʵ����ģ�ҵ�ϳɹ�����ص��������£�



