��Ŀ����
����Ŀ����ҵ���Ѿ�ʵ��CO2��H2��Ӧ�ϳɼ״�����һ���¡������ܱ������г���2molCO2��6molH2��һ�������·�����Ӧ��CO2(g)+3H2(g)=CH3OH(g)+H2O(g)�����CO2��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ����ش�

��1�����ܱ��������ݻ���___��
��2���ﵽδƽ��״̬��ʱ����___min(�3������10��)��
��3����ǰ3min�ڣ���H2Ũ�ȵı仯��ʾ�ķ�Ӧ����v(H2)=___mol/(L��min)��
��4��10minʱ��ϵ��ѹǿ�뿪ʼʱѹǿ֮��Ϊ__��
��5����ƽ���H2O(g)�����ʵ���������___��
��6����֪����CO(g)+2H2(g)=CH3OH(g) ��H=-90.1kJ/mol����CO(g)+H2O(g)=CO2(g)+H2(g����H=-41.1kJ/mol����CO2��H2��Ӧ�ϳ�CH3OH(g)���Ȼ�ѧ����ʽ__����Ӧ��10min�����ų�������Ϊ__��
���𰸡�2L 3 0.225 mol/(L��min) ![]() 30% CO2(g)+3H2(g)=CH3OH(g)+H2O(g) ��H =-49 kJ/mol 98 kJ
30% CO2(g)+3H2(g)=CH3OH(g)+H2O(g) ��H =-49 kJ/mol 98 kJ
��������
��һ���¡������ܱ������г���2molCO2��6molH2����ͼ���֪��CO2�ij�ʼŨ��Ϊ1mol/L�������= ![]() =2L���������ʵ�Ũ�Ȳ�����ʱ��ı仯���仯������Ӧ����ƽ��״̬����ͼ���֪��3min��CO2��CH3OH��Ũ�Ȼ��ڷ����仯��10min��CO2��CH3OH��Ũ�Ȳ������仯����3min��δƽ��״̬��10minΪƽ��״̬������v=
=2L���������ʵ�Ũ�Ȳ�����ʱ��ı仯���仯������Ӧ����ƽ��״̬����ͼ���֪��3min��CO2��CH3OH��Ũ�Ȼ��ڷ����仯��10min��CO2��CH3OH��Ũ�Ȳ������仯����3min��δƽ��״̬��10minΪƽ��״̬������v=![]() ���㷴Ӧ���ʣ���ͼ��֪���ڵ�3minʱ������v=
���㷴Ӧ���ʣ���ͼ��֪���ڵ�3minʱ������v=![]() ��֪������̼��Ӧ���ʣ�������̼������������֮�ȵ������ǵļ�����֮�ȣ�����������ķ�Ӧ���ʣ�
��֪������̼��Ӧ���ʣ�������̼������������֮�ȵ������ǵļ�����֮�ȣ�����������ķ�Ӧ���ʣ�
��4��10minʱ�ﵽƽ�⣬��������ʽ�����10min����������ʵ����뿪ʼʱ�����ʵ���֮�ȣ�����ͬ����£������ѹǿ֮�ȵ������ʵ���֮�ȣ��ɼ����10minʱ��ϵ��ѹǿ�뿪ʼʱѹǿ֮��Ϊ����ƽ���H2O(g)�����ʵ���������=nH2O/n�ܣ����ø�˹���ɢ�-�ڿɵõ���ȷ���Ȼ�ѧ����ʽ��
��1����һ���¡������ܱ������г���2molCO2��6molH2����ͼ���֪��CO2�ij�ʼŨ��Ϊ1mol/L�������=n/v=2/1=2L��
�ʴ�Ϊ��2L��
��2���������ʵ�Ũ�Ȳ�����ʱ��ı仯���仯������Ӧ����ƽ��״̬����ͼ���֪��3min��CO2��CH3OH��Ũ�Ȼ��ڷ����仯��10min��CO2��CH3OH��Ũ�Ȳ������仯����3min��δƽ��״̬��10minΪƽ��״̬��
�ʴ�Ϊ��3��
��3����ͼ��֪���ڵ�3minʱ������v=![]() ��֪������̼��Ӧ���ʣ�������̼������������֮�ȵ������ǵļ�����֮�ȣ�����������ķ�Ӧ���ʣ�����ͼ���֪��vCO2=
��֪������̼��Ӧ���ʣ�������̼������������֮�ȵ������ǵļ�����֮�ȣ�����������ķ�Ӧ���ʣ�����ͼ���֪��vCO2=![]() =
=![]() =0.075 mol/(L��min)�����ݷ�ӦCO2(g)+3H2(g)=CH3OH(g)+H2O(g)��������̼������������֮�ȵ���1��3����vCO2��vH2=1��3��vH2==3vCO2=3��0.075 mol/(L��min)=0.225 mol/(L��min)��
=0.075 mol/(L��min)�����ݷ�ӦCO2(g)+3H2(g)=CH3OH(g)+H2O(g)��������̼������������֮�ȵ���1��3����vCO2��vH2=1��3��vH2==3vCO2=3��0.075 mol/(L��min)=0.225 mol/(L��min)��
�ʴ�Ϊ��0.225 mol/(L��min)��
��4���г�����ʽ CO2(g)+3H2(g)=CH3OH(g)+H2O(g)
ʼ mol/L 1 3 0 0
�� mol/L 0.75 2.25 0.75 0.75
ƽ mol/L 0.25 0.75 0.75 0.75
10min��ﵽƽ�⣬10minʱ��ϵ��ѹǿ�뿪ʼʱѹǿ֮��Ϊ![]() =
=![]() ��
��
�ʴ�Ϊ��![]() ��
��
��5����ƽ���H2O(g)�����ʵ�������=![]() =
=![]() =30%��
=30%��
�ʴ�Ϊ��30%��
��6����֪����CO(g)+2H2(g)=CH3OH(g) ��H1=-90.1kJ/mol����CO(g)+H2O(g)=CO2(g)+H2(g����H2=-41.1kJ/mol�����ø�˹�ɢ�-�ڵõ�CO2(g)+3H2(g)=CH3OH(g)+H2O(g) ��H=��H1-����H2��=-90.1+41.1=-49 kJ/mol��1molCO2��ȫ��Ӧ���ɷų�49 kJ������������Ŀ������2molCO2��ȫ��Ӧ����ų�������98 kJ��������
�ʴ�Ϊ��CO2(g)+3H2(g)=CH3OH(g)+H2O(g) ��H =-49 kJ/mol��98 kJ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��2019����ʷ�������֯��ȫ��ƻ���12��4�շ������棺�о���ʾ��ȫ�������̼�ŷ�������������CO2���ۺ������ǽ�������������Ч;����
(1)CO2�������Ƽ״����йط�Ӧ�����ڲ�ͬ�¶��µĻ�ѧƽ�ⳣ�����±���ʾ��
��ѧ��Ӧ | ƽ�ⳣ�� | ||
500�� | 700�� | 800�� | |
��.H2(g)+CO2(g) H2O(g)+CO(g) | 1.0 | 1.70 | 2.52 |
��.2H2(g)+CO(g) CH3OH(g) | 2.5 | 0.34 | 0.15 |
��.3H2(g)+CO2(g) CH3OH(g)+H2O(g) ��H | |||
����H___0(������������������������)��
����֪��Ӧ�������ʷ���ʽ������=k����c3(H2)��c(CO2)������=k����c(CH3OH)��c(H2O)��k����k��Ϊ���ʳ�������Ӧ�ﵽƽ��������¶ȣ�k������ı���___k������ı���(��������������С��������������)��
��500��ʱ������ݵ��ܱ������м���1molCO2��1molH2�����Ʒ�Ӧ����ֻ������Ӧ�����ﵽƽ���ֻ�ı�������������ʹCO��ƽ����������������___(��ѡ����ĸ)��
A.����ѹǿ B.�����¶� C.��ͨ������ʵ���CO2��H2 D.���������ˮ
(2)��200��ʱ����5L����ѹ�Ƶĺ����ܱ�������ͨ��2molCO2��2molCH4������ӦCH4(g)+CO2(g)2H2(g)+2CO(g)����ó�ʼѹǿΪP0kPa����Ӧ��������������ѹǿ(P)��ʱ��(t)�仯(��Ӧ�ﵽƽ��ʱ���¶�����ʼ�¶���ͬ)��ͼ��ʾ��
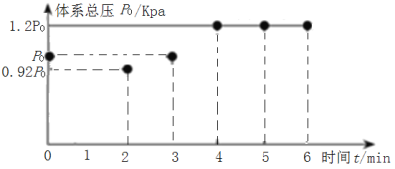
�ٸ÷�Ӧ�����д�0min��2minѹǿ�仯ԭ����___��
��0��4min�ڣ���Ӧ��ƽ����Ӧ������(CO2)=___��
����ƽ���ѹ����ƽ��Ũ�ȱ�ʾ�Ļ�ѧƽ�ⳣ��Kp=___��[�����ѹ(p��)=������ѹ(p��)�������������]
(3)��ѧ���������CO2��CH4�Ʊ����ϳ�����(CO��H2)���ܵķ�Ӧ������ͼ��ʾ��
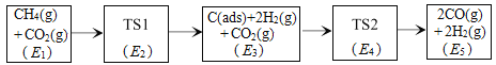
ע��C(ads)Ϊ�����Ի���̿�������ڰ��������༰��Ŀ���������������[���һ��������1��CH4(g)+1��CO2(g)�����������ΪE1eV����λ��eV]�����У�TS��ʾ����̬��
��CH4(g)+CO2(g) 2H2(g)+2CO(g)��H=___kJ��mol-1(��֪��1eV=1.6��10-22kJ)
����E4+E1��E3+E2��������Ʊ����ϳ�������Ӧ���ʵķ�Ӧ����ʽΪ___��