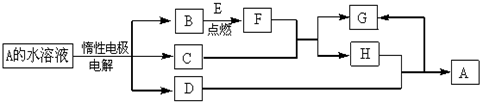
��Ŀ����
2����1��24mL 0.05mol•L-1��Na2SO3��Һǡ�ñ�20mL 0.02mol•L-1��K2R2O7��Һ��������Ԫ��R�ڻ�ԭ�����еĻ��ϼ���+3����2����0.64g Cuȫ������һ������Ũ�����У������������0.009mol����NO��NO2��N2O4��������������0.032mol�������ɵ������������ϣ���ͨ��NaOH��Һ�У��������ﱻ��ȫ���գ�����ֻ��NaNO3��H2O����������вμӷ�Ӧ��O2�ڱ�״���µ����Ϊ112mL��
���� ��1��Na2SO3������ΪNa2SO4��SԪ�ػ��ϼ���+4������Ϊ+6�ۣ�K2R2O7��XԪ�ط�����ԭ��Ӧ����RԪ���ڲ����еĻ��ϼ�Ϊa�ۣ����ݵ���ת���غ����a��ֵ��
��2���������⣺Cu��NxOy��NaNO3����ϵ����غ㣬�õ�Cu��O2�Ĺ�ϵʽ���㼴�ɣ�
��� ��������1����RԪ���ڲ����еĻ��ϼ�Ϊa�ۣ����ݵ���ת���غ㣬��24��10-3L��0.05mol/L����6-4��=20��10-3L��0.02mol/L��2����6-a�������a=+3��
�ʴ�Ϊ��+3��
��2���������⣺Cu��NxOy��NaNO3������NxOyʱHNO3���õ��ĵ�����������NaNO3ʱNxOy��ʧȥ�ĵ�������ȣ���ô��Cu��ʧȥ�ĵ�������O2���õ��ĵ�������Ȼ��ȣ���Cu��O2�Ĺ�ϵʽΪ��2Cu��O2��n��Cu��=0.01 mol������n��O2��=0.005 mol�������Ϊ112mL��
�ʴ�Ϊ��112��
���� ���⿼��������ԭ��Ӧ���㡢������ԭ��Ӧ����ȣ��Ѷ��еȣ���ʾ����ʧ��Ŀ�ǽ���ؼ����������յ���ת���غ�˼������ã�

��ϰ��ϵ�д�
�����Ŀ
12�����й���Cl2���ʵ�˵����ȷ���ǣ�������
| A�� | �ܶȱȿ���С | B�� | ����ɫ��ζ������ | ||
| C�� | ��ʹ�������ɫ������ɫ | D�� | ����NaOH��Һ��Ӧ |
13���������ӷ���ʽ��д��ȷ���ǣ�������
| A�� | ����������Һ��ϡ���ᷴӦ��Ba2++SO42-+H++OH-=BaSO4��+H2O | |
| B�� | ���۵⻯����Һ�ڿ����б�����4I-+O2+2H2O=4OH-+2I2 | |
| C�� | �����������������Fe2O3+6H+=2Fe3++3H2O | |
| D�� | �ð�ˮ����������SO2���壺OH-+SO2=HSO3- |
10����NAΪ�����ӵ���������ֵ������˵��������ǣ�������
| A�� | NA �������������3mol �� �� | |
| B�� | 18gˮ�к���NA �Թµ��Ӷ� | |
| C�� | NA �����������к���2mol �м� | |
| D�� | NA ��������̼�����к���2mol �м� |
14��������ʯī���õ������Ӻ���0.01mol/L ���Ȼ�����Һ�У�����˵����ȷ���ǣ�������
| A�� | ������������OH- | B�� | ������������ | ||
| C�� | ʯī���Ϸų����� | D�� | ʯī���Ϸų����� |
12����ʾ���б仯�Ļ�ѧ������ȷ���ǣ�������
| A�� | ��ϩ�ķ���ʽ��CH2=CH2 | |
| B�� | NaHCO3�ĵ��룺HCO3-+H2O?H3O++CO32- | |
| C�� | NaOH�Ľṹʽ��Na-O-H | |
| D�� | NH4Cl�ĵ���ʽ��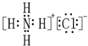 |
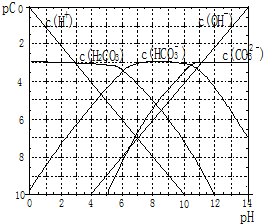 pC����pH����ָ��ϡ��Һ���������ʵ���Ũ�ȵij��ö�����ֵ����ij��Һ���ʵ�Ũ��Ϊ��1��10-3mol/L�������Һ�и����ʵ�pC=-lg1��10-3=3����֪H2CO3��Һ�д��ڵĻ�ѧƽ��Ϊ��CO2+H2O?H2CO3?H++HCO3-?2H++CO32-����ͼΪH2CO3��Һ��pC-pHͼ����ش��������⣺
pC����pH����ָ��ϡ��Һ���������ʵ���Ũ�ȵij��ö�����ֵ����ij��Һ���ʵ�Ũ��Ϊ��1��10-3mol/L�������Һ�и����ʵ�pC=-lg1��10-3=3����֪H2CO3��Һ�д��ڵĻ�ѧƽ��Ϊ��CO2+H2O?H2CO3?H++HCO3-?2H++CO32-����ͼΪH2CO3��Һ��pC-pHͼ����ش��������⣺