��Ŀ����
18��ͭ���仯��������ѧ��ѧ����Ҫѧϰ���ݣ���1����Ȼ���и���ԭ��ͭ�����ᆳ�������������ú�ת��Ϊ����ͭ��Һ�������ܲ���������������п��ZnS���ͷ�Ǧ��PbS����������ת��Ϊͭ����CuS����
����ͭ��������������������������ͭ���ù��̵Ļ�ѧ����ʽΪCuS+2O2 $\frac{\underline{\;һ������\;}}{\;}$CuSO4��
������������������ͭ��Һ������п������Ӧ�����ӷ���ʽΪCu2+��aq��+ZnS��s��=CuS��s��+Zn2+��aq�������Ҫ˵���÷�Ӧ�ܷ�����ԭ����һ�������£��ܽ��С�Ŀ������ת��Ϊ�ܽ�ȸ�С�Ŀ��
��2����ҵ�����û�ͭ����Ҫ�ɷ���Cu2S��ұ��ͭ��Ϊ�˲ⶨ��ͭ����Ʒ�Ĵ��ȣ��ɽ���Ʒ����������Ը��������Һ��Ӧ���÷�Ӧ�����ӷ���ʽΪCu2S+2MnO4-+8H+=2Cu2++SO42-+2Mn2++4H2O��
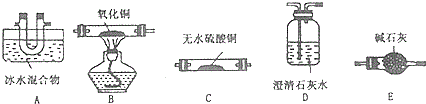
��3����ҵ�Ͽ��ö��ַ����Ʊ����ᾧ�壮��ͼ�����÷�ͭм�������������Ʊ�����������ͭ���壩������ͼ��
��֪��
| ��Һ�б��������� | Fe3+ | Fe2+ | Cu2+ |
| ��ȫ���������������ʱ����Һ��pH | ��3.7 | ��6.4 | ��4.4 |
����ҺB�к��е���������Fe2+��Fe3+��H +��Cu2�������ӷ��ţ���������X��ѡ�õ�������H2O2���ѧʽ����
�ڼ����Լ�Y��Ϊ�˵���pH���Լ�Y����ѡ�����CuO��CuCO3��Cu��OH��2��
�۲���Z�IJ���������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����ȣ�
�ܽ���ͭмͶ�뵽���ᣨ�����ᡢ���ᣩ�п����Ʊ�����ͭ���壬��ij100mL������Һ�У�c��HNO3��=2mol•L-1��c��H2SO4��=4mol•L-1�������������������������÷���������������Ʊ�����ͭ���壨CuSO4•5H2O��������Ϊ75g��
������һ�麬��ͭ�̵�ͭƬ�����費���������ʣ��ڿ��������տ���������ͭ�����ʣ����ⶨ����Ӧǰ������������ͬ�����ͭƬ��ͭ��������Ϊ34%[��֪������������=��������Ľ�������/����������������100%]��
���� ��1���ٸ�����Ŀ��Ϣ�Լ������غ���д��ѧ����ʽ��
�ڸ�����ͭ�ܽ��С����п������ͭ��Һ������п��������ת����Ӧ������ͭ��
��2����ͭ����Ҫ�ɷ���Cu2S�������Ը��������Һ��Ӧ����ͭ���ӣ���������ӣ����и���������ӱ���ԭΪ�����ӣ����ԭ���غ�͵���غ���ƽ��д��
��3���٢ڢ����Ʊ�ʵ�����̿�֪��Fe��Cu���գ�ͭ��������Ӧ����CuO��Fe����������Ӧ������ϡ���ᣬ��Ӧ��������ͭ���������������������Ჿ��������������Ϊ�˳�ȥ������������ȫ���������������ʱ����Һ��pH��֪�轫����������������������Ȼ�����pH�γɳ��������˳�ȥ����������������Һ������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����ɵõ������ݴ˽��
��������Һ�����������ʵ�����������������ʵ�����������������ʵ�������Ϸ�Ӧ3Cu+8H++2NO3-=3Cu2++2NO��+4H2O�����Ӷ�����ϵ��Ԫ���غ���������
�����ݻ�ѧ����ʽ���պ��������䣬��Ӧ���ĵ����������ɵĶ�����̼��ˮ��������ͬ��Ȼ����ݻ�ѧ����ʽ��Ͻ���������=$\frac{������Ľ�������}{������������}$��100%����õ���
��� �⣺��1������ͭ��������������������������ͭ����Ӧ�Ļ�ѧ����ʽΪ��CuS+2O2 $\frac{\underline{\;һ������\;}}{\;}$CuSO4 ��
�ʴ�Ϊ��CuS+2O2 $\frac{\underline{\;һ������\;}}{\;}$CuSO4 ��
������������������ͭ��Һ������п����һ�������£��ܽ��С�Ŀ������ת��Ϊ�ܽ�ȸ�С�Ŀ����ͭ�ܽ���С����п����������ת����Ӧ�����ӷ���ʽ��Cu2+��aq��+ZnS��s��=CuS��s��+Zn2+��aq����
�ʴ�Ϊ��Cu2+��aq��+ZnS��s��=CuS��s��+Zn2+��aq������һ�������£��ܽ��С�Ŀ������ת��Ϊ�ܽ�ȸ�С�Ŀ��
��2����ͭ����Ҫ�ɷ���Cu2S�������Ը��������Һ��Ӧ����ͭ���ӣ���������ӣ����и���������ӱ���ԭΪ�����ӣ���Ӧ�����ӷ���ʽΪ��Cu2S+2MnO4-+8H+=2Cu2++SO42-+2Mn2++4H2O��
�ʴ�Ϊ��Cu2S+2MnO4-+8H+=2Cu2++SO42-+2Mn2++4H2O��
��3���٢ڢ����Ʊ�ʵ�����̿�֪��Fe��Cu���գ�ͭ��������Ӧ����CuO��Fe����������Ӧ������ϡ���ᣬ��Ӧ��������ͭ���������������������Ჿ����������������ʱ��Һ�к���������Fe2+��Fe3+��H +��Cu2��Ϊ�˳�ȥ������������ȫ���������������ʱ����Һ��pH��֪�轫����������ͨ��˫��ˮ������������������ͨ��CuO��CuCO3��Cu��OH��2����pH�γɳ��������˳�ȥ����������������Һ������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����ɵõ������ݴ˽��
�ʴ�Ϊ����Fe2+��Fe3+��H +��Cu2��H2O2��
��CuO��CuCO3��Cu��OH��2��
������Ũ������ȴ�ᾧ��
��ij����c��HNO3��=2mol•L-1��c��H2SO4��=4mol•L-1�ķ�����Һ100mL�����������������������n��H+��=0.1L��2mol/L+0.1L��4mol/L��2=1mol��n��NO3-��=0.1L��2mol/L=0.2mol��n��SO42-��=0.1L��4mol/L=0.4mol��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O�����ݶ�����ϵ��֪���������ȫ����Ӧ����������0.8mol������ͭ0.3mol��������������ͭ�������Ϊ0.3mol��������Ʊ�����ͭ���壨CuSO4•5H2O��������=0.3mol��250g/mol=75g��
�ʴ�Ϊ��75��
�ݺ���ͭ�̵�ͭƬ�����費���������ʣ��ڿ�������������ȫ��Ӧ�����ⶨ����Ӧǰ������������ͬ��˵����Ӧ�����������������ɵĶ�����̼��ˮ������������ͬ����ͭ����ͭ�����ʵ���Ϊx��ͭ�̵����ʵ���Ϊy��
2Cu+O2 $\frac{\underline{\;\;��\;\;}}{\;}$ 2CuO��
x 0.5x
Cu2��OH��2CO3$\frac{\underline{\;\;��\;\;}}{\;}$2CuO+CO2+H2O
y y y
�ɷ�Ӧ���ĵ����������ɵĶ�����̼��ˮ��������ͬ����0.5x��32=18y+44y�����x��y=31��8
����Ԫ���غ���㣬����������=$\frac{������Ľ�������}{������������}$��100%=$\frac{2y��64}{��2y+x����64}$��100%=$\frac{2��\frac{8}{31}x��64}{��2��\frac{8}{31}x+x����64}$��100%=34%��
�ʴ�Ϊ��34%��
���� ���⿼����ͭ���仯�������ʷ�������ѧ����ʽ���㣬�����Ʊ���ԭ���ͼ�������ݻ����ǹؼ�����Ŀ�Ѷ��еȣ�

| A�� | SO${\;}_{4}^{2-}$��Ħ��������96g/mol | |
| B�� | 1molH2���2molH | |
| C�� | 1molH2O����������NA��H2O�������ܺ� | |
| D�� | 1molO2������Ϊ32g/mol |
 ��������һ����Ҫ�����ȼ�ϣ�Ҳ�������������������ȣ��Գ��������ƻ����ã���ҵ�Ͽ�����ú���������ˮú�����ϳɶ����ѣ���ش��������⣺
��������һ����Ҫ�����ȼ�ϣ�Ҳ�������������������ȣ��Գ��������ƻ����ã���ҵ�Ͽ�����ú���������ˮú�����ϳɶ����ѣ���ش��������⣺��1��ú����������Ҫ��ѧ��Ӧ����ʽΪ��C+H2O$\frac{\underline{\;����\;}}{\;}$CO+H2��
��2������ˮú���ϳɶ����ѵ�������Ӧ���£�
��2H2��g��+CO��g��?CH3OH��g����H=-90.8kJ•mol-1
��2CH3OH��g��?CH3OCH3��g��+H2O��g����H=-23.5kJ•mol-1
��CO��g��+H2O��g��?CO2��g��+H2��g����H=-41.3kJ•mol-1
д��ˮú��ֱ�Ӻϳɶ�����ͬʱ����CO2���Ȼ�ѧ��Ӧ����ʽ3H2��g��+3CO��g��?CH3OCH3��g��+CO2��g������H=-246.4 kJ•mol-1��
��3��һ�������µ��ܱ������У����ܷ�Ӧ�ﵽƽ�⣬Ҫ���CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ��bce��
a�����µ�ѹ ������������b�����¸�ѹ
c������CO2��Ũ�� ������d������CO��Ũ��
e������������� �������� f���������
��4����֪��Ӧ��2CH3OH��g��?CH3OCH3��g��+H2O��g��ij�¶��µ�ƽ�ⳣ��Ϊ400�����¶��£����ܱ������м���CH3OH����Ӧ��ijʱ�̲�ø���ֵ�Ũ�����£�
| ���� | CH3OH | CH3OCH3 | H2O |
| Ũ��/��mol•L-1�� | 0.44 | 0.6 | 0.6 |
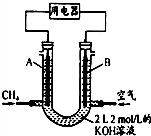
��5����CH4��Ƴ�ȼ�ϵ�أ���װ��ʾ����ͼ��A��BΪ�����̼��������ͨ����飬�ڱ�״���£����ļ������VL��
��0��V��44.8Lʱ������ܷ�Ӧ����ʽΪCH4+2O2+2KOH=K2CO3+3H2O��
��44.8L��V��89.6Lʱ�������缫��ӦΪCH4-8e-+9CO32-+3H2O=10HCO3-��
��V=67.2Lʱ����Һ��������Ũ�ȴ�С��ϵΪc��HCO3-����c��CO32-����c��OH-����
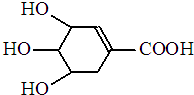 �˽����㺬��һ�ֿ����������в�������Ҫ�ɷ�-ç���ᣬ����ӽṹ��ͼ��ʾ�����й���ç�����˵��������ǣ�������
�˽����㺬��һ�ֿ����������в�������Ҫ�ɷ�-ç���ᣬ����ӽṹ��ͼ��ʾ�����й���ç�����˵��������ǣ�������| A�� | ������ˮ | B�� | ��ʹ���Ը��������Һ��ɫ | ||
| C�� | �ܷ���������Ӧ | D�� | ��FeCl3��Һ����ɫ |
| A�� | CH2=CH2+H-OH$\frac{\underline{����}}{��}$CH3-CH2-OH | |
| B�� | CH2=CH-CH=CH2+2H2$\frac{\underline{\;����\;}}{\;}$CH3-CH2-CH2-CH3 | |
| C�� | 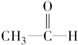 +H2$\stackrel{����}{��}$CH3-CH2-OH +H2$\stackrel{����}{��}$CH3-CH2-OH | |
| D�� | CH3-CH3+2Cl2$\stackrel{����}{��}$CH2Cl-CH2Cl+2HCl |
 +2C2H5OH
+2C2H5OH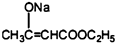 +CH3COOH��
+CH3COOH��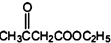 +CH3COONa
+CH3COONa
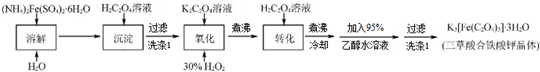
 ijͬѧ�Ը�����Ϊԭ����ˮ��һ����һˮ��ѭ��������ȡ���Ტ̽���ⶨ���ᾧ�壨H2C2O4•xH2O����ijЩ���ʣ�ͨ���������Ͽ�֪������������ˮ����һ����Ҫ�Ļ���ԭ�ϣ��㷺����ҩ���������߷��Ӻϳɵȹ�ҵ�����ᾧ�����ȵ�100��ʱʧȥ�ᾧˮ����Ϊ��ˮ���ᣮ157��ʱ��������������ʼ�ֽ⣻���������ڵ����¿�����Ϊ���壻����Ʋ�����ˮ������������ʹ����ʯ��ˮ����ǣ�
ijͬѧ�Ը�����Ϊԭ����ˮ��һ����һˮ��ѭ��������ȡ���Ტ̽���ⶨ���ᾧ�壨H2C2O4•xH2O����ijЩ���ʣ�ͨ���������Ͽ�֪������������ˮ����һ����Ҫ�Ļ���ԭ�ϣ��㷺����ҩ���������߷��Ӻϳɵȹ�ҵ�����ᾧ�����ȵ�100��ʱʧȥ�ᾧˮ����Ϊ��ˮ���ᣮ157��ʱ��������������ʼ�ֽ⣻���������ڵ����¿�����Ϊ���壻����Ʋ�����ˮ������������ʹ����ʯ��ˮ����ǣ�