��Ŀ����
3�� ijͬѧ�Ը�����Ϊԭ����ˮ��һ����һˮ��ѭ��������ȡ���Ტ̽���ⶨ���ᾧ�壨H2C2O4•xH2O����ijЩ���ʣ�ͨ���������Ͽ�֪������������ˮ����һ����Ҫ�Ļ���ԭ�ϣ��㷺����ҩ���������߷��Ӻϳɵȹ�ҵ�����ᾧ�����ȵ�100��ʱʧȥ�ᾧˮ����Ϊ��ˮ���ᣮ157��ʱ��������������ʼ�ֽ⣻���������ڵ����¿�����Ϊ���壻����Ʋ�����ˮ������������ʹ����ʯ��ˮ����ǣ�
ijͬѧ�Ը�����Ϊԭ����ˮ��һ����һˮ��ѭ��������ȡ���Ტ̽���ⶨ���ᾧ�壨H2C2O4•xH2O����ijЩ���ʣ�ͨ���������Ͽ�֪������������ˮ����һ����Ҫ�Ļ���ԭ�ϣ��㷺����ҩ���������߷��Ӻϳɵȹ�ҵ�����ᾧ�����ȵ�100��ʱʧȥ�ᾧˮ����Ϊ��ˮ���ᣮ157��ʱ��������������ʼ�ֽ⣻���������ڵ����¿�����Ϊ���壻����Ʋ�����ˮ������������ʹ����ʯ��ˮ����ǣ�
�����������Ϣ�ش��������⣺
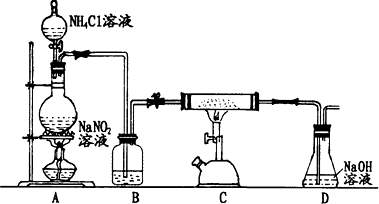
��1��ͼʾ�٢ڵ�����һˮ�����������ͼ1��װ���н��еģ�ָ��װ��B���������������ܣ�
��2��ͼʾ�٢ڵ�����һˮ������У���������������Ӧ��ʱ�����������ͬ������£��ı䷴Ӧ�¶��Կ��췴Ӧ�¶ȶԲ����ռ��ʵ�Ӱ�죬�����ͼ2��ʾ����ѡ����ѵķ�Ӧ�¶�Ϊ70�森
��3����������װ�����һ��̽������֤������ʵ��װ�ã����ᾧ��ֽ�װ���ԣ����ӵ�����ȥ����
��ش��������⣺
��װ�õ�����˳��Ϊ��A��C��D��E��B��D��
��ʵ��ʱ���ڵ�ȼB���ƾ���֮ǰ��Ӧ���еIJ������ռ�D�����壬���ھƾ��ƻ��沿�����������ۡ����������ٵ�ȼ�ƾ��ƣ����ռ�D�����壬����CO�Ĵ��ȣ���
������װ�ô��ڲ�����֮��Ϊû��һ����̼β������װ�ã�
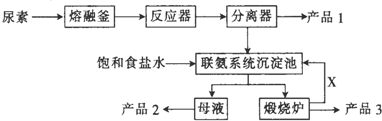
��4�����������ڹ�ҵ������Ҫ���ã���������Ʊ������������������£�
��ȡFeSO4•7H2O������С�ձ��У�����ˮ������ϡH2SO4��Һ�ữ�������ܽ⣮�����Һ�м���һ������H2C2O4��Һ���������Һ�������У����Ͻ��裬���Ⱪ�У����л�ɫ�����������������ã������������ˮ����Һ���ټ�������ˮ�������ȣ����ˣ����ϴ�ӳ��������ˣ��ñ�ͪϴ�ӹ����������ɣ�����ͪ������ˮ���ӷ���
�����ɵIJ�����������ϴ�ӳ����������Ƿ�ϴ����ȫ�ķ�����ȡ���һ��ϴ��Һ1��2mL�����Թ��У������еμ������ữ��BaCl2��Һ�����ް�ɫ�����������������ϴ�Ӹɾ���
���ñ�ͪϴ�ӹ������ε�Ŀ���ǽ��Ͳ����������ܽ�����ͬʱ����ϴȥ�����ˮ�֣�
���� ��1�����������Ľṹ�ص��жϣ�BΪ���������ܣ������ӷ��������������
��2�������¶���������������ж���ѷ�Ӧ�¶ȣ�
��3���ٲ��������ΪCO��CO2��H2O��������100��ʱ��ʼ������157��ʱ��������������ʼ�ֽ⣮����Ʋ�����ˮ������������ʹ����ʯ��ˮ����ǡ����������ڵ����¿�����Ϊ���壬������֪�������ӷ����IJ��ᣬ�ټ���ˮ���������ɣ����������̼�����ɣ�����ȥ������̼����ͨ�����ȵ�����ͭ����һ����̼����Ĵ��ڣ����÷�Ӧ�����ɵĶ�����̼ͨ�����ʯ��ˮ�����֤��һ����̼�Ĵ��ڣ�
��CO�ǿ�ȼ�����壬�������ϼ��Ȼ��ȼ�ᷢ����ը��
��CO�ж�����Ⱦ��������Ҫβ������װ�ã�
��4�����жϳ����Ƿ�ϴ���ķ�����ȡ���һ��ϴ�ӵ���Һ�������Һ���Ƿ����δϴ�Ӹɾ������ʣ������в����������Ʊ�����FeSO4•7H2O������С�ձ��м���ˮ������ϡH2SO4��Һ�ữ�������ܽ⣬�������������ܻ���ϡH2SO4���ʣ����Ӧ�ü������һ��ϴ����Һ���Ƿ���SO42-���������ת����SO42-�ļ��飻
���ñ�ͪϴ�����β����ɣ���ͪ�ӷ����������������ڱ�ͪ�������ڸ��������ʧ��
��� �⣺��1����������ϡ������ݣ�ˮ��õ������ǣ��ټ���ϡ������������õ����ᣬ��A������ƿ�з�����Ӧ��BΪ���������ܣ������ӷ��������������ᣬ
�ʴ�Ϊ�����������ܣ�
��2������ͼ3��֪�����¶�Ϊ70��ʱ��������ռ�����ߣ�����ѡ�����ѷ�Ӧ�¶���70�棻
�ʴ�Ϊ��70�棻
��3���ٲ��������ΪCO��CO2��H2O��������100��ʱ��ʼ������157��ʱ��������������ʼ�ֽ⣬����Ʋ�����ˮ������������ʹ����ʯ��ˮ����ǡ����������ڵ����¿�����Ϊ���壬������֪�������ӷ����IJ��ᣬ�ټ���ˮ���������ɣ����������̼�����ɣ�����ȥ������̼����ͨ�����ȵ�����ͭ����һ����̼����Ĵ��ڣ����÷�Ӧ�����ɵĶ�����̼ͨ�����ʯ��ˮ�����֤��һ����̼�Ĵ��ڣ�װ������˳��Ϊ��A��C��D��E��B��D��
�ʴ�Ϊ��C��E��
��CO�ǿ�ȼ�����壬�������ϼ��Ȼ��ȼ�ᷢ����ը��������ҪCװ���г���CO���ٵ�ȼ�ƾ��Ƽ��ȣ�����ʵ��ʱ���ڵ�ȼB���ƾ���֮ǰ��Ӧ���еIJ����ǣ��ռ�D�����壬���ھƾ��ƻ��沿�����������ۡ����������ٵ�ȼ�ƾ��ƣ����ռ�D�����壬����CO�Ĵ��ȣ���
�ʴ�Ϊ���ռ�D�����壬���ھƾ��ƻ��沿�����������ۡ����������ٵ�ȼ�ƾ��ƣ����ռ�D�����壬����CO�Ĵ��ȣ���
��CO�ж�����Ⱦ����������ֱ���ſգ�����Ҫ��β������װ�ã�
�ʴ�Ϊ��û��һ����̼β������װ�ã�
��4���ٱ����в����������Ʊ�����FeSO4•7H2O������С�ձ��м���ˮ������ϡH2SO4��Һ�ữ�������ܽ⣬�������������ܻ���ϡH2SO4���ʣ����Ӧ�ü������һ��ϴ����Һ���Ƿ���SO42-���������Ϊ��ȡ���һ�ε�ϴ����Һ1��2 mL ���Թ��У������еμ��������ữ��BaCl2��Һ�����ް�ɫ�����������������ϴ�Ӹɾ���
�ʴ�Ϊ��ȡ���һ��ϴ��Һ1��2mL�����Թ��У������еμ������ữ��BaCl2��Һ�����ް�ɫ�����������������ϴ�Ӹɾ���
�ڱ�ͪ�ӷ����������������ڱ�ͪ�����Ͳ����������ܽ�����ͬʱ����ϴȥ�����ˮ�֣�
�ʴ�Ϊ�����Ͳ����������ܽ�����ͬʱ����ϴȥ�����ˮ�֣�
���� ���⿼�����Ʊ�ʵ�鷽����ƣ��漰��ѧʵ�顢���ʵ����ʣ���Ŀ�Ѷ��еȣ�ע������̽����������ʵ�鷽����Ƶķ�������ȷ�������ʼ�ʵ���������Ϊ���ؼ����������������ѧ���ķ�����������������ѧʵ��������

| A�� | ���½ṹ�մ����������ǽ������� | |
| B�� | �������������;���ĺϽ𣬷�Ϊ̼�ظֺͺϽ�� | |
| C�� | ��оƬ�Ǹ��ּ���������Ӳ�Ʒ�ĺ��IJ��� | |
| D�� | �����賣����������ά |
��1����Ȼ���и���ԭ��ͭ�����ᆳ�������������ú�ת��Ϊ����ͭ��Һ�������ܲ���������������п��ZnS���ͷ�Ǧ��PbS����������ת��Ϊͭ����CuS����
����ͭ��������������������������ͭ���ù��̵Ļ�ѧ����ʽΪCuS+2O2 $\frac{\underline{\;һ������\;}}{\;}$CuSO4��
������������������ͭ��Һ������п������Ӧ�����ӷ���ʽΪCu2+��aq��+ZnS��s��=CuS��s��+Zn2+��aq�������Ҫ˵���÷�Ӧ�ܷ�����ԭ����һ�������£��ܽ��С�Ŀ������ת��Ϊ�ܽ�ȸ�С�Ŀ��
��2����ҵ�����û�ͭ����Ҫ�ɷ���Cu2S��ұ��ͭ��Ϊ�˲ⶨ��ͭ����Ʒ�Ĵ��ȣ��ɽ���Ʒ����������Ը��������Һ��Ӧ���÷�Ӧ�����ӷ���ʽΪCu2S+2MnO4-+8H+=2Cu2++SO42-+2Mn2++4H2O��
��3����ҵ�Ͽ��ö��ַ����Ʊ����ᾧ�壮��ͼ�����÷�ͭм�������������Ʊ�����������ͭ���壩������ͼ��
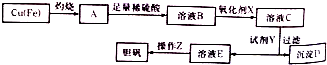
��֪��
| ��Һ�б��������� | Fe3+ | Fe2+ | Cu2+ |
| ��ȫ���������������ʱ����Һ��pH | ��3.7 | ��6.4 | ��4.4 |
����ҺB�к��е���������Fe2+��Fe3+��H +��Cu2�������ӷ��ţ���������X��ѡ�õ�������H2O2���ѧʽ����
�ڼ����Լ�Y��Ϊ�˵���pH���Լ�Y����ѡ�����CuO��CuCO3��Cu��OH��2��
�۲���Z�IJ���������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����ȣ�
�ܽ���ͭмͶ�뵽���ᣨ�����ᡢ���ᣩ�п����Ʊ�����ͭ���壬��ij100mL������Һ�У�c��HNO3��=2mol•L-1��c��H2SO4��=4mol•L-1�������������������������÷���������������Ʊ�����ͭ���壨CuSO4•5H2O��������Ϊ75g��
������һ�麬��ͭ�̵�ͭƬ�����費���������ʣ��ڿ��������տ���������ͭ�����ʣ����ⶨ����Ӧǰ������������ͬ�����ͭƬ��ͭ��������Ϊ34%[��֪������������=��������Ľ�������/����������������100%]��
| A�� | 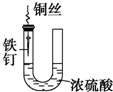 �����ױ���ʴ | |
| B�� | 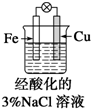 �μ�����KSCN��Һ����Һ��ΪѪ��ɫ | |
| C�� | 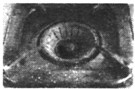 ȼ��������IJ�λ�������⣬��Ҫ�����ڸ�������������ѧ��ʴ | |
| D�� |  ������þ��ķ�������ֹ���¸����ܵ��ĸ�ʴ��þ���൱��ԭ��ص����� |
| A�� | һ���¶Ⱥ�ѹǿ�£�������̬��������Ĵ�С���ɹ�������ķ��Ӵ�С������ | |
| B�� | ����Ħ�������ָ1Ħ���κ�������ռ�����ԼΪ22.4L�� | |
| C�� | ��ͬ�����壬�������ͬ�������������ķ�����Ҳ��ͬ | |
| D�� | һ���¶Ⱥ�ѹǿ�£�������̬��������Ĵ�С���ɹ�������ķ��������� |