��Ŀ����
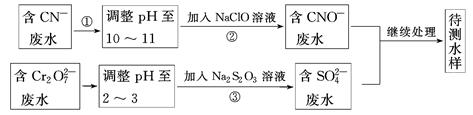
Ǧ��ұ�����¹������£�
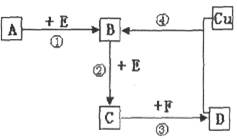
�ٸ���������Ǧ��(PbS)���и�ѡ���ڱ��գ�2PbS��3O2 2PbO��2SO2�����ƴ�Ǧ��PbO��C
2PbO��2SO2�����ƴ�Ǧ��PbO��C Pb��CO����PbO��CO
Pb��CO����PbO��CO Pb��CO2��
Pb��CO2��
����˵����ȷ����(����)
�ٸ���������Ǧ��(PbS)���и�ѡ���ڱ��գ�2PbS��3O2
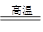 2PbO��2SO2�����ƴ�Ǧ��PbO��C
2PbO��2SO2�����ƴ�Ǧ��PbO��C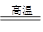 Pb��CO����PbO��CO
Pb��CO����PbO��CO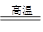 Pb��CO2��
Pb��CO2������˵����ȷ����(����)
| A����ѡ��������Ǧ��Ĺ������ڻ�ѧ�仯 |
| B����Ǧ���շ�Ӧ�У�PbS�ǻ�ԭ������ԭ����ֻ��PbO |
| C������ұ�������У���ȡ1 mol Pb��ת��2 mol���� |
| D����l mol PbS��ȫұ����Pb������������Ҫ6 g̼ |
D
������Ǧ��Ĺ������������仯��A����B���л�ԭ�����PbO���SO2������ұ�������У���ȡ1 mol Pb��ת��8 mol���ӣ�C����

��ϰ��ϵ�д�
�����Ŀ
 (NxOy)�� H2O(��ƽʱx��y�þ�����ֵ��ʾ����������
(NxOy)�� H2O(��ƽʱx��y�þ�����ֵ��ʾ���������� CO(NH2)2 (l��+ H2O (l)��
CO(NH2)2 (l��+ H2O (l)��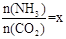 ����ͼ�ǰ�̼�ȣ�x����CO2ƽ��ת���ʣ������Ĺ�ϵ��������x����������ԭ���� ��
����ͼ�ǰ�̼�ȣ�x����CO2ƽ��ת���ʣ������Ĺ�ϵ��������x����������ԭ���� ��
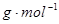 �� ��Ӧ�ٵĻ�ѧ����ʽΪ ��
�� ��Ӧ�ٵĻ�ѧ����ʽΪ ��