��Ŀ����
��27.2 g Cu��Cu2O�Ļ�����м���ijŨ�ȵ�ϡ����0.5 L������������ȫ��Ӧ������NO��Cu(NO3)2����������Һ�м���1.0 mol��L��1��NaOH��Һ1.0 L����ʱ��Һ�����ԣ�������������ȫ��������������Ϊ39.2 g�������й�˵������ȷ���ǣ� ��
| A��Cu��Cu2O�����ʵ���֮��Ϊ2��1 |
| B����������ʵ���Ũ��Ϊ2.6 mol��L��1 |
| C��������NO�ڱ�״���µ����Ϊ4.48 L |
| D��Cu��Cu2O�����ᷴӦ��ʣ��HNO3Ϊ0.2 mol |
B
��Cu��Cu2O�����ʵ����ֱ�x��y
��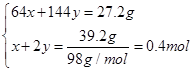
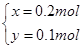
A����ȷ��C�2x��2y��3n(NO)��n(NO)��0.2 mol��C����ȷ��D�ʣ��HNO3Ϊ1.0 mol��L��1��1.0 L��0.4 mol��2��0.2 mol����ȷ��n(HNO3)��1.0 mol��L��1��1.0 L��0.2 mol��1.2 mol
c(HNO3)��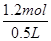 ��2.4 mol��L��1��
��2.4 mol��L��1��
��
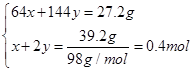
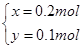
A����ȷ��C�2x��2y��3n(NO)��n(NO)��0.2 mol��C����ȷ��D�ʣ��HNO3Ϊ1.0 mol��L��1��1.0 L��0.4 mol��2��0.2 mol����ȷ��n(HNO3)��1.0 mol��L��1��1.0 L��0.2 mol��1.2 mol
c(HNO3)��
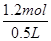 ��2.4 mol��L��1��
��2.4 mol��L��1��
��ϰ��ϵ�д�
�����Ŀ
 2PbO��2SO2�����ƴ�Ǧ��PbO��C
2PbO��2SO2�����ƴ�Ǧ��PbO��C
