��Ŀ����
A��B��C��D��F�dz����Ļ��������F�ڳ�������һ����ɫҺ�壬DΪǿ�ᣬ�������ͼת����ϵ����Ӧ���������ֲ�������ȥ�����ش��������⣺

��1����A��B��C��D��Ϊ����Ԫ�صĻ����A��һ�ֳ����Ŀ�ʯ����Ҫ�ɷ֣���A��Ħ������Ϊ120 �� ��Ӧ�ٵĻ�ѧ����ʽΪ ��
�� ��Ӧ�ٵĻ�ѧ����ʽΪ ��
��2����A��B��C��D��Ϊ����Ԫ�صĻ������A��һ��������ֻ����10�����ӣ���
��A����ʽΪ__________��
�ڷ�Ӧ�ܵ����ӷ���ʽΪ________________________________________________
��ȡCu��Cu2O�Ļ������Ʒ12��0g�����뵽������D��ϡ��Һ�У�����ˮ���ռ����������壬��״���������Ϊ2��24L������Ʒ��Cu2O������Ϊ__________g��

��1����A��B��C��D��Ϊ����Ԫ�صĻ����A��һ�ֳ����Ŀ�ʯ����Ҫ�ɷ֣���A��Ħ������Ϊ120
 �� ��Ӧ�ٵĻ�ѧ����ʽΪ ��
�� ��Ӧ�ٵĻ�ѧ����ʽΪ ����2����A��B��C��D��Ϊ����Ԫ�صĻ������A��һ��������ֻ����10�����ӣ���
��A����ʽΪ__________��
�ڷ�Ӧ�ܵ����ӷ���ʽΪ________________________________________________
��ȡCu��Cu2O�Ļ������Ʒ12��0g�����뵽������D��ϡ��Һ�У�����ˮ���ռ����������壬��״���������Ϊ2��24L������Ʒ��Cu2O������Ϊ__________g��
��1��4FeS2+11O2 Fe2O3+8SO2����2�֣�
Fe2O3+8SO2����2�֣�
��2����NH3 ����2�֣�
��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O����2�֣�
��4��32g��2�֣�4��3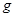 Ҳ�÷֣�
Ҳ�÷֣�
 Fe2O3+8SO2����2�֣�
Fe2O3+8SO2����2�֣���2����NH3 ����2�֣�
��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O����2�֣�
��4��32g��2�֣�4��3
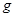 Ҳ�÷֣�
Ҳ�÷֣������������1��A��һ�ֳ����Ŀ�ʯ����Ҫ�ɷ֣���A��Ħ������Ϊ120
 ��A��FeS2,EΪ��������Ӧ�ٵĻ�ѧ����ʽΪ4FeS2+11O2
��A��FeS2,EΪ��������Ӧ�ٵĻ�ѧ����ʽΪ4FeS2+11O2 Fe2O3+8SO2��
Fe2O3+8SO2����2����A��B��C��D��Ϊ����Ԫ�صĻ����A��һ��������ֻ����10�����ӣ���A��NH3��
��E������,B��NO��C��NO2��F��ˮ��DΪ���ᣬ���Է�Ӧ�ܵ����ӷ���ʽΪ3Cu+8H++2NO3-=3Cu2++2NO��+4H2O
����Cu��Cu2O�����ʵ����ֱ�Ϊx��y�����ݵ�ʧ�����غ㣬2��24L/22��4L/mol��3=2x+2y,���ߵ�����Ϊ12��0g��������64x+144y=12�����y=0��03mol,���Cu2O��������0��03mol��144g/mol=4��32g

��ϰ��ϵ�д�
 �����߿����ϵ�д�
�����߿����ϵ�д� �㾦�½̲�ȫ�ܽ��ϵ�д�
�㾦�½̲�ȫ�ܽ��ϵ�д� Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�
�����Ŀ

 2PbO��2SO2�����ƴ�Ǧ��PbO��C
2PbO��2SO2�����ƴ�Ǧ��PbO��C

