��Ŀ����
(14��)��������Ԫ�أ�����A��B��C��DΪ����������Ԫ�أ�E��FΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش����⡣
��1��A��̬ԭ����������ߵĵ��ӣ���������ڿռ��� ������ԭ�ӹ���� �Ρ�
��2��ijͬѧ����������Ϣ��������B�����Ų�ͼ��ͼ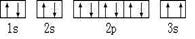 ��Υ���� ԭ����
��Υ���� ԭ����
��3��Fλ�� �� �������̬ԭ���� ���˶�״̬��
��4��CD3 ����ԭ�ӵ��ӻ���ʽΪ ���ü۲���ӶԻ��������Ʋ�����ӿռ乹��Ϊ ������EԪ�صķ����� ��
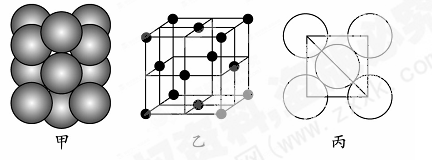
��5����ij�������ʾ�����ԭ�ӵĶѻ���ʽ����ͼ����ʾ���侧����������ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ����ͼ����ʾ�����и�ԭ�ӵ���λ��Ϊ ���õ��ʾ�����ԭ�ӵĶѻ���ʽΪ���ֻ����ѻ���ʽ�е� ������֪�ý�����ԭ�Ӱ뾶Ϊd cm��NA���������ӵ����������������ԭ������ΪM����þ�����ܶ�Ϊ______g��cm��3(����ĸ��ʾ)��
| AԪ��ԭ�ӵĺ���p����������s����������1 |
| BԪ��ԭ�Ӻ���s����������p����������ȣ��Ҳ���AԪ����ͬһ���� |
| Cԭ�Ӻ�������p���ȫ������� |
| DԪ�ص������������������IJ�Ϊ4 |
| E��ǰ�������е縺����С��Ԫ�� |
| F�����ڱ��ĵ����� |
��2��ijͬѧ����������Ϣ��������B�����Ų�ͼ��ͼ
 ��Υ���� ԭ����
��Υ���� ԭ������3��Fλ�� �� �������̬ԭ���� ���˶�״̬��
��4��CD3 ����ԭ�ӵ��ӻ���ʽΪ ���ü۲���ӶԻ��������Ʋ�����ӿռ乹��Ϊ ������EԪ�صķ����� ��
��5����ij�������ʾ�����ԭ�ӵĶѻ���ʽ����ͼ����ʾ���侧����������ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ����ͼ����ʾ�����и�ԭ�ӵ���λ��Ϊ ���õ��ʾ�����ԭ�ӵĶѻ���ʽΪ���ֻ����ѻ���ʽ�е� ������֪�ý�����ԭ�Ӱ뾶Ϊd cm��NA���������ӵ����������������ԭ������ΪM����þ�����ܶ�Ϊ______g��cm��3(����ĸ��ʾ)��

��1��3���Ĵ��λ������� ��2������������ ��3���������ڵڢ�B��d��25
��4��sp3�������Σ���ɫ��Ӧ ��5��12��ͭ�ͻ����������ѻ���
��4��sp3�������Σ���ɫ��Ӧ ��5��12��ͭ�ͻ����������ѻ���
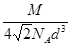
���������A��B��C��D��EΪ����������Ԫ�أ�ԭ��������������AԪ��ԭ�ӵĺ���p��������s��������1�������Ų�Ϊ1s22s22p3����AΪN��BԪ��ԭ�Ӻ���s����������p����������ȣ��Ҳ���AԪ����ͬһ���ڣ���������Ų�Ϊ1s22s22p63s2����BΪMg��Cԭ�Ӻ�������p���ȫ������������ԭ��������֪���۵���Ϊ3s23p3�������⣬��CΪP��DԪ�ص������������������IJ�Ϊ4��ԭ����������P����DΪ�������ڵڢ�A��Ԫ�أ���DΪCl��E��ǰ�������е縺����С��Ԫ�أ���EΪK��F�����ڱ��ĵ����У���FΪMn����
��1��AΪN��������ߵĵ���Ϊ2p���ӣ���������ڿռ���3������p���Ϊ�Ĵ��Σ�
��2��������ԭ����֪��������ͬһ����ڵ���������Ӧ�෴��B��̬ԭ�ӵĺ�������Ų�ͼ��3s�ϵ�������������������ͬ����Υ��������ԭ����
��3��FΪMn���ڵ������ڵڢ�B���������Ϊd���ӣ���d�������������Ϊ25����25���˶�״̬��ͬ�ĵ��ӣ�
��4��PCl3��Pԭ�ӹµ��Ӷ���Ϊ1���ɼ���Ϊ3����Ϊsp3�ӻ����ռ乹��Ϊ�����Σ�FΪK������KԪ��Ӧ������ɫ��Ӧ��
��5���ɾ����ṹ��֪���Զ���ԭ���о�����֮�����ԭ�Ӵ��������ϣ�ÿ������ԭ��Ϊ12���湲�ã��ʾ����и�ԭ�ӵ���λ��Ϊ12���õ��ʾ�����ԭ�ӵĶѻ���ʽΪ���������ѻ����ɾ����ṹ��֪��������ԭ�ӵ���ĿΪ8��
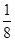 +6��
+6��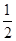 =4���þ�����ԭ�ӵ�����=4��
=4���þ�����ԭ�ӵ�����=4��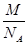 g������Ϣ��֪����ͼ��֪Ϊ����������ԭ�Ӱ뾶Ϊdcm����ͼ����֪���������ⳤ="4d" cm��
g������Ϣ��֪����ͼ��֪Ϊ����������ԭ�Ӱ뾶Ϊdcm����ͼ����֪���������ⳤ="4d" cm��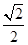 =2
=2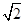 d cm���ʾ��������=��2
d cm���ʾ��������=��2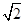 d cm��3=16
d cm��3=16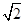 d3 cm3���������ܶ�=
d3 cm3���������ܶ�=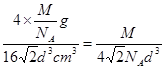 g/cm3��
g/cm3��
��ϰ��ϵ�д�
�����Ŀ

 ��������� �� ���� �� ��֮��Ϊ
��������� �� ���� �� ��֮��Ϊ 



