��Ŀ����
��.����˵������ȷ���� ��
E��CH4��BCl3��CO2���Ǻ��м��Լ��ķǼ��Է���
��.������������һ����Ȼ�粻���ڵ��˹��ϳɳ�Ӳ���ϣ�Ӳ�Ƚ����ڽ��ʯ���dz�Ӳ�������������Ҫ�ɾ�֮һ����ش��������⣺
(1)�ڵڶ����ڣ�ԭ�ӵĵ�һ������һ����˵������������� ���Ƚ�����ԭ�ӵĵ�һ�����ܣ�Be B��N O(�������<��)����ԭ���� ��
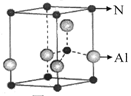
(2)��ͼΪ����������ľ���������Ļ�ѧʽΪ ���þ�����Bԭ�����Nԭ�ӵ� ��϶�����þ����ı߳�Ϊa cm����ô�þ�����ܶ�Ϊ g/cm3(ֻҪ���г���ʽ)��

(3)����������ľ���ṹ����ʯ�Ľṹ���ƣ������۵�Ƚ��ʯ�ĵͣ��Է�����ԭ�� ��
(4)�����������������������ڸ��¸�ѹ���Ʊ��������������ֳơ���ʯī�����ṹ������������ʯī���ƣ�������������Nԭ�ӵ��ӻ��������Ϊ ���뻭�������������ƽ��ṹʾ��ͼ(�á��𡱴���Nԭ�ӣ��á�����Bԭ�ӣ�ÿ��ԭ�Ӳ�����7��)��
| A��SO2��O3��PO43-��ClO4-��Ϊ�ȵ����� |
| B���������ʧȥһ��H�����γ�CH3-����̼ԭ�ӵ��ӻ����ͷ����˸ı� |
| C��Tiԭ�ӵĺ�������Ų�ʽΪ[Ar]3d34s2 |
| D��CS2��H2O��C2H2����ֱ���η��� |
��.������������һ����Ȼ�粻���ڵ��˹��ϳɳ�Ӳ���ϣ�Ӳ�Ƚ����ڽ��ʯ���dz�Ӳ�������������Ҫ�ɾ�֮һ����ش��������⣺
(1)�ڵڶ����ڣ�ԭ�ӵĵ�һ������һ����˵������������� ���Ƚ�����ԭ�ӵĵ�һ�����ܣ�Be B��N O(�������<��)����ԭ���� ��
(2)��ͼΪ����������ľ���������Ļ�ѧʽΪ ���þ�����Bԭ�����Nԭ�ӵ� ��϶�����þ����ı߳�Ϊa cm����ô�þ�����ܶ�Ϊ g/cm3(ֻҪ���г���ʽ)��

(3)����������ľ���ṹ����ʯ�Ľṹ���ƣ������۵�Ƚ��ʯ�ĵͣ��Է�����ԭ�� ��
(4)�����������������������ڸ��¸�ѹ���Ʊ��������������ֳơ���ʯī�����ṹ������������ʯī���ƣ�������������Nԭ�ӵ��ӻ��������Ϊ ���뻭�������������ƽ��ṹʾ��ͼ(�á��𡱴���Nԭ�ӣ��á�����Bԭ�ӣ�ÿ��ԭ�Ӳ�����7��)��
(��)AE
(��)(1)���� �� �� Beԭ����2s�������ȫ����״̬��Nԭ����2p������ڰ����״̬���DZȽ��ȶ���״̬�������ǵĵ�һ�����ܸ����������ڵ�ԭ��
(2)BN ������

(3)�������������е�����ļ����Ƚ��ʯ������̼̼���ļ���Ҫ�������۵�Ƚ��ʯ�ĵ�
(4)sp2
(��)(1)���� �� �� Beԭ����2s�������ȫ����״̬��Nԭ����2p������ڰ����״̬���DZȽ��ȶ���״̬�������ǵĵ�һ�����ܸ����������ڵ�ԭ��
(2)BN ������


(3)�������������е�����ļ����Ƚ��ʯ������̼̼���ļ���Ҫ�������۵�Ƚ��ʯ�ĵ�
(4)sp2
(��)����ʧȥ�����ӣ����ӻ�����û�з����仯��Ti�Ļ�̬ԭ�Ӻ�������Ų�Ϊ[Ar]3d24s2��ˮ�Ǣ��η��ӡ�ͬһ����Ԫ�صĵ�һ����������ԭ�������������������Ҳ�������������ڶ����ڣ�Be��B��N��O������Beȫ������N������Ľṹ�ȶ��йء�
(��)��������������ľ����ṹͼ��֪���þ����к���4��Bԭ�ӡ�4��Nԭ�ӣ��ʵ�����ķ���ʽΪBN��Bԭ�����ڵ�ԭ�ӵ���������ṹ�У�ÿһ�����������Ϊa3 cm3�������ӵ����������������ΪNA a3 cm3��BN������Ϊ24.8 g/mol��4mol��99.2 g��BN���ܶ�Ϊ g/cm3��BN�۵�Ƚ��ʯ�͵�ԭ���뻯ѧ����ǿ���йأ�B��N���ļ�������C��C���ļ���������������ṹ��������ʯī���ƣ����B��Nԭ�Ӿ�����sp2�ӻ�����ƽ��ṹʾ��ͼ����ԭ�ӽ�����֡�
g/cm3��BN�۵�Ƚ��ʯ�͵�ԭ���뻯ѧ����ǿ���йأ�B��N���ļ�������C��C���ļ���������������ṹ��������ʯī���ƣ����B��Nԭ�Ӿ�����sp2�ӻ�����ƽ��ṹʾ��ͼ����ԭ�ӽ�����֡�
(��)��������������ľ����ṹͼ��֪���þ����к���4��Bԭ�ӡ�4��Nԭ�ӣ��ʵ�����ķ���ʽΪBN��Bԭ�����ڵ�ԭ�ӵ���������ṹ�У�ÿһ�����������Ϊa3 cm3�������ӵ����������������ΪNA a3 cm3��BN������Ϊ24.8 g/mol��4mol��99.2 g��BN���ܶ�Ϊ
 g/cm3��BN�۵�Ƚ��ʯ�͵�ԭ���뻯ѧ����ǿ���йأ�B��N���ļ�������C��C���ļ���������������ṹ��������ʯī���ƣ����B��Nԭ�Ӿ�����sp2�ӻ�����ƽ��ṹʾ��ͼ����ԭ�ӽ�����֡�
g/cm3��BN�۵�Ƚ��ʯ�͵�ԭ���뻯ѧ����ǿ���йأ�B��N���ļ�������C��C���ļ���������������ṹ��������ʯī���ƣ����B��Nԭ�Ӿ�����sp2�ӻ�����ƽ��ṹʾ��ͼ����ԭ�ӽ�����֡�
��ϰ��ϵ�д�
�����Ŀ