��Ŀ����
14�� ij��Һֻ�����������ӣ�NH4+��Na+��Fe2+��NO3-��I-��SO32-��AlO2-�е����֣�����ˮ�ĵ��룩���Ҹ����ӵ����ʵ���Ũ����ȣ��ֽ�������ʵ�飺
ij��Һֻ�����������ӣ�NH4+��Na+��Fe2+��NO3-��I-��SO32-��AlO2-�е����֣�����ˮ�ĵ��룩���Ҹ����ӵ����ʵ���Ũ����ȣ��ֽ�������ʵ�飺��ȡ��������Һ��NaOH��Һ���ȣ������̼�����ζ�����壬δ�۲쵽������������
����ȡ��������Һ�����������ᣬ�ɹ۲쵽����ɫ���壬δ�۲쵽�������ɣ�
��������ʵ�飬����˵����ȷ���ǣ�������
| A�� | ��ʵ���ֻ��ȷ��ԭ��Һ��һ����NH4+��û��Fe2+�� | |
| B�� | ȡʵ��ں����Һ�μӵ�����Һ�����ܱ���ɫ�� | |
| C�� | ԭ��Һ�п��ܺ���NH4+��Na+��SO32-��I-�������� | |
| D�� | ȡ����ԭ��Һ�����Ը��������Һ���������������ӱ����� |
���� ��ȡ��������Һ��NaOH��Һ���ȣ������̼�����ζ�����弴ΪNH3������Һ�к�NH4+������NH4+�����������ӣ��ܺ�AlO2-����˫ˮ����������δ�۲쵽��������������Һ����Fe2+��
����ȡ��������Һ�����������ᣬ�ɹ۲쵽����ɫ���壬˵����Һ���л�ԭ�����ӣ���I-��SO32-���л�������һ�֣��ݴ˷�����
��� �⣺ij��Һֻ�����������ӣ�NH4+��Na+��Fe2+��NO3-��I-��SO32-��AlO2-�е����֣�����ˮ�ĵ��룩���Ҹ����ӵ����ʵ���Ũ����ȣ�
��ȡ��������Һ��NaOH��Һ���ȣ������̼�����ζ�����壬��NH3������Һ�к�NH4+������NH4+�����������ӣ��ܺ�AlO2-����˫ˮ�⣬����Һ����AlO2-��δ�۲쵽��������������Һ����Fe2+��
����ȡ��������Һ�����������ᣬ�ɹ۲쵽����ɫ���壬˵����Һ���л�ԭ�����ӣ���I-��SO32-���л�������һ�֣�
����Һ��һ����Fe2+��AlO2-��һ����NH4+��I-��SO32-���л�������һ�֣�������Na+��NO3-��
��������Ҫ����4�֣��Ҹ����ӵ����ʵ���Ũ����ȣ�������ҺҪ�������غ��֪����Һ�в��ܴ���SO32-������Һ����SO32-ʱ����һ������Na+��NH4+�����ܺ�NO3-��I-����ʱ��Һ�ж����������4�֣�����������Ҫ������Һ��һ����I-������Ҫ��4��������Ũ����ȣ�����Һ��һ����Na+��NH4+��I-��NO3-��
A����ʵ��ٳ�����ȷ��ԭ��Һ��һ����NH4+��û��Fe2+����������֮��Ļ����ԣ�����ȷ��һ������AlO2-����A����
B��������Һ�к�I-���ʼ���������������ܽ�������ΪI2���ʼ�����ۺ��������B��ȷ��
C��������Һ�ĵ���غ��֪����Һ�в��ܴ���SO32-����C����
D��������Һ�к�Na+��NH4+��I-��NO3-���ʼ�����������Һ��ֻ��I-����������D����
��ѡB��
���� ���⿼�������ӵļ��飬�������ӹ������Լ���Һ�ĵ��������������ۺ��Խ�ǿ���ѶȽϴ�

 ��У����ϵ�д�
��У����ϵ�д�| A�� | ��������������� | B�� | Ư���� | ||
| C�� | ������ | D�� | ��ԭ�� |
| A�� | ��A-x+m��mol | B�� | ��A-x-m��mol | C�� | $\frac{W}{A}$��A-x+m��mol | D�� | $\frac{W}{A}$��A-x-m��mol |

 ��
��

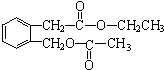 +2NaOH$\stackrel{��}{��}$
+2NaOH$\stackrel{��}{��}$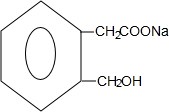 +CH3COONa+CH3CH2OH+H2O����Ӧ������ˮ�ⷴӦ����ȡ����Ӧ����
+CH3COONa+CH3CH2OH+H2O����Ӧ������ˮ�ⷴӦ����ȡ����Ӧ���� ����д��������
�����������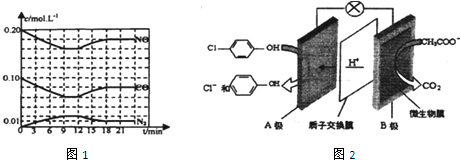
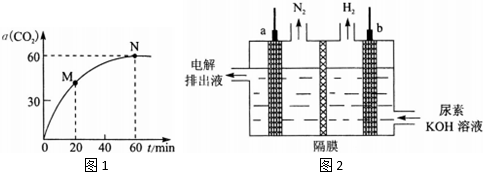
 �������Է�ˮ�������������ط���ȥ����ԭ����ͼ2��ʾ
�������Է�ˮ�������������ط���ȥ����ԭ����ͼ2��ʾ