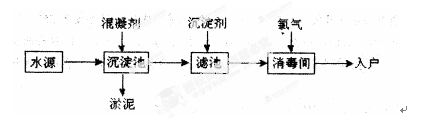
��Ŀ����
ѧУ�����ĺ�ˮ�и�Ƽ�賤������ˮ�ʶ���ˮˮ���п��ܺ���Fe3����Ba2����K����H+��NO3����Cl-��CO32-��SO42-���ӡ�Ϊ�˽�һ��ȷ�ϣ�ȡ������ʵ���⣺
��ȡˮ����ϸ�۲죬��������һ״̬��
����pH��ֽ�ⶨ��ˮ��pH����ֽ�Ժ�ɫ��
����ˮ���е���KSCN��Һ���ʺ�ɫ��
����ˮ���е���ϡ���ᣬ�д�����ɫ�����������ټ�ϡ���ᣬ��ɫ��������ʧ��
��1���ɴ˿�֪������ˮ�п϶����е�������_________���϶�û�е�������_________��
��2�� ��Ƽ�賤�Ŀ���ԭ����ˮ�к��н϶��_____________���ӡ�
��1��Fe3����Ba2����H+�� CO32-��SO42-�� ��2��K����NO3��
���������������pH��ֽ�ⶨ��ˮ��pH����ֽ�Ժ�ɫ��˵��ˮ�������ԣ�����H+��CO32-��Ϊ����H+��Ӧ�����ڣ�������ˮ���е���KSCN��Һ���ʺ�ɫ��֤������Fe3��������CO32-��Fe3����CO32-�ᷢ�����ӷ�Ӧ����ˮ���е���ϡ���ᣬ�д�����ɫ�����������ټ�ϡ���ᣬ��ɫ��������ʧ��֤��ԭ��Һ�к���Ba2������CO32-��SO42- �����Ƕ���ͱ����ӷ�����Ӧ��ʹˮ�����ǣ�������Ŀ��ì�ܡ���ԭ��Һһ������Fe3����Ba2����H+һ������CO32-��SO42-��ֲ��������Ҫ������N��P��KԪ�أ����ˮ���������ӣ��ڸ�ˮ���и�Ƽ�賤�Ŀ���ԭ����ˮ�к��н϶��K����NO3�����ӡ�
���㣺�������ӹ��桢���ӷ�Ӧ��֪ʶ��

ij��Һ�н����±������е�5�����ӣ�������ˮ�ĵ��뼰���ӵ�ˮ�⣩�������ӵ����ʵ�����Ϊ1mol��
| ������ | SO42-��NO3-��Cl- |
| ������ | Fe3+��Fe2+��NH4+��Cu2+��Al3+ |
������ԭ��Һ�м���KSCN��Һ�������Ա仯��������ԭ��Һ�м�����������ᣬ���������ɣ���Һ������������䡣��������Һ�м���BaCl2��Һ���а�ɫ���������Իش��������⡣
��1��������ԭ��Һ�м�����������ᣬ�ټ���KSCN��Һ�������� ��
��2��ԭ��Һ�к��е��������� ��
��3����ԭ��Һ�м������������ᣬ������Ӧ�����ӷ���ʽΪ ��
��4����ԭ��Һ�м���������NaOH��Һ����ַ�Ӧ���ˡ�ϴ�ӡ����գ��������ù�����������ƽ��������Ϊ ��
ij���ͭ�������ַ�ˮ��Ҫ������һ�ַ�ˮ�к���CN-���ӣ���һ�ַ�ˮ�к���Cr2O72-���ӣ��ó��ⶨ��ͼ��ʾ�ķ�ˮ�������̡�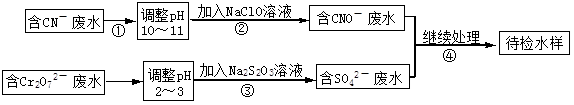 �ش��������⣺
�ش��������⣺
��1������ڷ�����Ӧ�����ӷ���ʽ�ɱ�ʾ���£�aCN��+bClO��+2cOH��=dCNO��+eN2��+fCO32��+bCl��+cH2O���������ӷ���ʽ���ܵ���ƽϵ���ж��飬��ش�
�ٷ���ʽ��e : f��ֵΪ ����ѡ���ţ���
| A��1 | B��1/2 | C��2 | D������ȷ�� |
������Ӧ��ת��0.6mol���ӣ������ɵ������ڱ���µ������ ��
��2��������з�Ӧʱ��ÿ0.4molCr2O72-ת��2.4mol�ĵ��ӣ��÷�Ӧ�����ӷ���ʽΪ ��
��3��ȡ��������ˮ�����Թ��У��ȼ���NaOH��Һ���۲쵽����ɫ�������ɣ���������NaOH��Һ��ֱ�����ٲ�����ɫ����Ϊֹ���ټ���Na2S��Һ���к�ɫ�������ɣ�����ɫ�������٣� �������ӷ���ʽ��ʾ����������ɫ�仯��ԭ��
�ٲ�����ɫ���������ӷ���ʽΪ ���ں��ֱ��ɫ���������ӷ���ʽΪ ��
��4��ͭ���������ϵ�dz����е���ɫ��������֪�����£�����Һ��Cu2+�ȶ���Cu+�������������·�����2Cu+=" Cu+" Cu2+�������+1��ͭ�Ļ�������������磺Cu2O��CuI��CuCl��CuH�ȡ�
��д��CuH�ڹ���ϡ���������������ɵ����ӷ���ʽ ��
�ڽ�CuH�ܽ���������ϡ�����У�������з�Ӧ�Ļ�ѧ����ʽ:
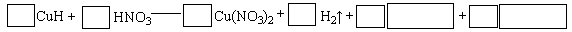
��1.52 g��ͭþ�Ͻ���ȫ�ܽ���50mL14.0 mol/L��Ũ�����У��õ�NO2��N2O4�Ļ������1120mL(��״��)����Ӧ�����Һ�м���1.0 mol/L NaOH��Һ������������ȫ������ʱ���õ�2.54 g����������˵������ȷ����
| A������Ũ������ܶ�Ϊ1.40g/mL���Ũ�����������������Ϊ63% |
| B���úϽ���ͭ��þ�����ʵ���֮����2:1 |
| C��NO2��N2O4�Ļ�������У�NO2�����������80% |
| D���õ�2.54 g����ʱ������NaOH��Һ�������620 mL |