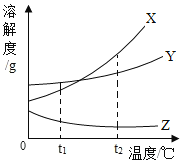
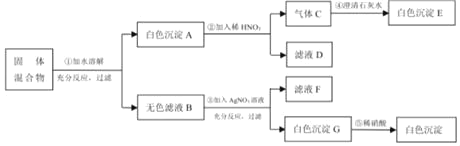
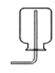
��Ŀ����
����Ŀ��ͭþ�Ͻ������ɻ����ߵȵ�����ϡ����ⶨ�Ͻ����ɣ�����Ԫ�غ��Բ��ƣ�������������ʵ�飺ȡ��3g�Ͻ���Ʒ����60gϡ�����Ϊ6�ȷ����μ�����Ʒ�У���ַ�Ӧ����ˡ�ϴ�ӡ�������أ��õ���ʵ���������£�
ϡ�������� | ��1�� | ��2�� | ��3�� | ��4�� | ��5�� | ��6�� |
ʣ��������� | 2.5g | m | 1.5g | 1.0g | 0.6g | 0.6g |
��1��ͭþ�Ͻ�����_____����������������
��2������ϡ���ᷢ����Ӧ�Ļ�ѧ����ʽΪ_____��
��3���������ݿ�֪������m��_____��
��4���Ͻ���Ʒ��ͭ������������_____��
��5������Ӧ�����в����������ռ��������ɵõ�����������Ϊ����g��_____
���𰸡������ Mg+H2SO4��MgSO4+H2�� 2 20% 0.2g
��������
����þ�������Ͷ�Ӧ�Ļ�ѧ����ʽ�������ɵ��������������Ͻ����ɽ����ͽ���������ͷǽ����ۺ϶��ɵľ��н������Ե����ʣ����ڻ���
��1���Ͻ����ڻ�������ͭþ�Ͻ����� ����
��2������ϡ���ᷢ����Ӧ��þ�����ᷴӦ��������þ����������Ӧ�Ļ�ѧ����ʽΪ Mg+H2SO4��MgSO4+H2����
��3��ǰ�Ĵ�ÿ�μ������ᶼ�ǹ��嶼�Ǽ�����0.5g�����Է������ݿ�֪������m��2��
��4���Ͻ���Ʒ��ͭ������������![]() ��100%��20%��
��100%��20%��
��5���μӷ�Ӧ��þ������Ϊ3g0.6g=2.4g
��ɵõ�����������Ϊx
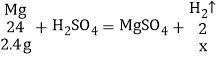
![]()
x��0.2g
������Ӧ�����в����������ռ��������ɵõ�����������Ϊ0.2g��

 ����ѧ����ϵ�д�
����ѧ����ϵ�д�����Ŀ����ѧ��ȤС���ͬѧ����ʵ��̨��һƿ��ǩ�������ɫ��Һ����ǩ��ͼ�������˽��֪����ɫ��Һԭ���������Һ�Ĺ�����ɴ˲²������_____��
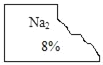
�������������̼���ơ�̼�����ơ������� �Ȼ��ơ���������Һ�е�һ�֣����Ǿ�������ƿ��Һ����ʵ��̽����
�����۷�����Ϊ��ȷ������Һ�������Ƚ����˷�����С��ͬѧ���ݱ�ǩ����������Ϊһ������_____�����ǵ�������_____��
���������ϣ�
Na2CO3+HCl��NaCl+NaHCO3 Na2CO3+2HCl��2NaCl+H2O+CO2��
NaHCO3+HCl��NaCl+H2O+CO2�� Na2CO3+BaCl2��BaCO3��+2NaCl
���̽����
Ϊȷ����ɷ֣�С��ͬѧ������·�������̽����̽��1��
ʵ�鲽�� | ���� | ���� |
����Һ�������Թ��У��μӹ�����BaCl2��Һ���� | ��_____ | �����Һ��Na2SO4��Һ |
���ˣ��������μ�ϡ���� | ��_____ |
��2��С����Ϊ������Ƹ���ʵ�鷽����̽��2��
ʵ�鲽�� | ���� | ���� |
ȡ����ɫ��Һ�������Թ��У������μ����������� | û�����ݲ��� | ��Һ��û��̼���ƣ������Һ��Na2SO4��Һ |
�����ͣ�����Ϊ������λͬѧ��ʵ�����ж��Ƿ���ȷ����˵������_____��
��ʵ�鷴˼���������ж����ʳɷ�ʱ�����������ʱ����������⣬���迼��_____��