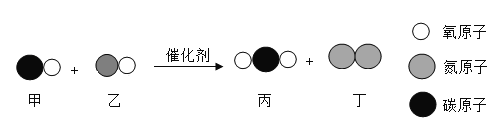
��Ŀ����
����Ŀ��ʵ��������һƿ������ˮ��̼�������Ʒ��Ϊ�ⶨ����̼����淋�����������ij��ѧ��ȤС��������ͼ��ʾװ�ý���ʵ�飨��װ�����������ã�װ��B��C����װҩƷ����������ʯ���������ƺ��������ƵĻ�������֪��̼����������ֽ⣬��Ӧ�Ļ�ѧ����ʽΪ��NH4HCO3![]() NH3��+H2O��+CO2����
NH3��+H2O��+CO2����
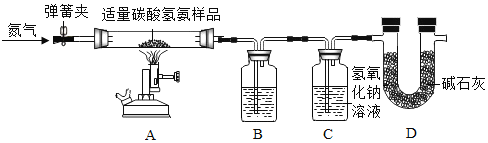
����ʵ�鲽�����£�
�������ɼУ�ͨ��һ��ʱ��ĵ�����
�����رյ��ɼУ���̼�������Ʒ���ȣ�
��������Ʒ��Ӧ��ȫ��������ֹͣ���ȣ�ֱ����������ȴ��
����ʵ����ϣ����װ��B��C��ҩƷ�������ֱ�������m1��m2
��ش��������⣺
��1��NH4HCO3����______��ѡ�������������������ط����������Ϸ�������װ��B��ʢ�ŵ�Һ����______��װ��D��������______��
��2��װ��C�з�����Ӧ�Ļ�ѧ����ʽΪ______��
��3���������У�ֹͣ����ǰӦ���еIJ�����______��
��4������Ʒ��̼����淋����������ı���ʽΪ______����m1��m2�Ĵ���ʽ��ʾ����
���𰸡����� Ũ���� ���տ����е�ˮ�Ͷ�����̼����ֹ������ˮ�Ͷ�����̼��ʵ������Ӱ�� 2NaOH+CO2�TNa2CO3+H2O ���ɼУ�ͨ��һ��ʱ��ĵ��� ![]()
��������
��1��NH4HCO3�Ǻ��е�Ԫ�صĻ��ʣ����ڵ��ʣ�װ��B��ʢ�ŵ�Һ����Ũ���ᣬ��������ˮ�����Ͱ�����װ��D�����������տ����е�ˮ�Ͷ�����̼����ֹ������ˮ�Ͷ�����̼��ʵ������Ӱ�졣
������ʣ�Ũ������տ����е�ˮ�Ͷ�����̼��
��2��װ��C�ж�����̼���������Ʒ�Ӧ����̼���ƺ�ˮ��������Ӧ�Ļ�ѧ����ʽΪ��2NaOH+CO2�TNa2CO3+H2O��
���2NaOH+CO2�TNa2CO3+H2O��
��3��������У�ֹͣ����ǰӦ���еIJ����Ǵ��ɼУ�ͨ��һ��ʱ��ĵ�����ʹ��Ӧ���ɵ�����ȫ�������ա�
������ɼУ�ͨ��һ��ʱ��ĵ�����
��4��ʵ����ϣ����װ��B��C��ҩƷ�������ֱ�������m1��m2��˵����Ʒ����Ϊ��m1+m2����Ӧ���ɶ�����̼��������m2��
��̼���������Ϊx��
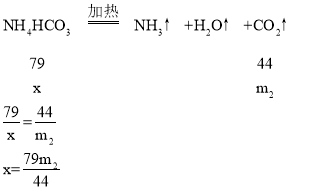
����Ʒ��̼����淋���������Ϊ��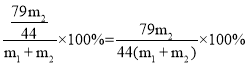
���![]() ��
��

����Ŀ��ij��ѧ��ȤС���ͬѧ��ͨ����ѯ��ʦ��������������Һ��Ũ���ᷴӦ���Ʊ�һ������SO2��Na2SO3+H2SO4��Ũ��=Na2SO4+SO2��+H 2O������ʦ�������ṩ��һƿ���õ�����������Һ����ȤС���ͬѧ�ֳɼס�����С��Ը�ƿ����������Һ�ɷֽ���ʵ��̽����
���������ϣ�
�� Na2SO3���ȶ����ڿ������ױ�����������Na2SO4��
��SO32-��SO42-������Ba2+��Ӧ������ɫ������
��BaSO3 ��������������ϡ���ᷴӦ���䷴Ӧԭ���� BaCO3��̼������ϡ���ᷴӦ���ơ�
(1)��������⣩�ٸ�ƿ��Һ�����ʵijɷ���ʲô��
�ڸ�ƿ��Һ���������Ƶ����������Ƕ��٣�
���������⣩ Na2SO3���ʵ�ԭ���ǣ�_______________________�����û�ѧ����ʽ��ʾ��
(2) ���������룩
����1��û�б��ʣ��ɷ���Na2SO3��
���� 2����ȫ���ʣ��ɷ���Na2SO4��
����3��_____________��
��ʵ��̽�������ס�������ֱ����ʵ��̽����Һ�Ƿ���ʣ�
С�� | ʵ����� | ���� | ���� |
���� | ȡ������Ʒ���Թ��м������ϡ��� | �������� | û�б��ʣ�����Na2SO3 |
���� | ȡ������Ʒ���Թ��м����Ȼ�����Һ���ټ�������ϡ���ᡣ | ������ɫ������ ________ | �Ѳ��ֱ��� |
����ͬѧʵ���з����Ļ�ѧ����ʽΪ��________________________����ͬѧ���ɼ��鷽����������������_________________��
(3) ��ʵ��̽�����������������ʵ��װ�òⶨNa2SO3��Һ��������������������![]() ��
��

��Ҫ�ⶨNa2SO3��Һ����������������ʵ������Ҫ��õ�����������������Һ��Ʒ�������Լ�_______������
��Dװ�õ�����Ϊ______________________________��