��Ŀ����
����Ŀ���������Ļ������ڹ�ũҵ�������й㷺��Ӧ�á�
��һ������Ӧ�úͷ���
1�����������Ҫ����Ϊ��
![]()
��1��д��ת���ڵĻ�ѧ����ʽ_______________��
��2��ת��������Fe2O3��xH2O����x��_____��xΪ��������
2���������ֹ�������һ����ʩ_______________��
���������Ļ�����Ӧ��
��������Ҫ�ɷ���FeS2������һ����Ҫ�Ļ���ԭ�ϣ��������Ʊ������������

��1������ҵ�Ͻ�����������Ŀ����______________��
��2��������������β���к���SO2����ֱ���ŷſ��ܻ���ɻ���������________��
��3��������¯�з�����Ӧ�Ļ�ѧ����ʽΪ___________��
��4����150t��FeS2 80%�Ļ������������������Ƶ�98%��Ũ����_________t��
����������ұ����̽��
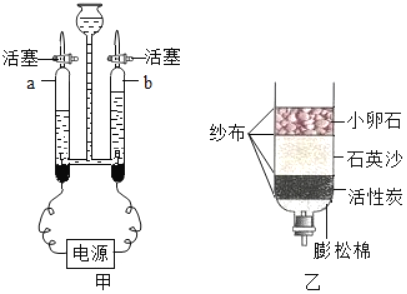
ȡ24.0g Fe2O3��ĩ��С��ͬѧ����ͼװ��ģ�����������ⶨ��Ӧ�����ɷ֡�
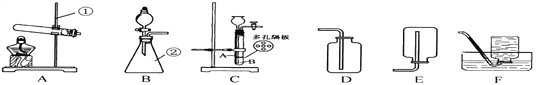
���ϣ���ʯ�ҿ�����H2O��CO2��
1������װ�ã���__________________����װ��ҩƷ��
2��ʵ��ʱ��ͨ��CO��Ŀ����__________________��
3����1������A���¶���700��������ȫ����ڣ�����ͨCO����ȴ��Ŀ���dz��˷�ֹ���ɵ�Fe���������__________________________________��
��2��ͨ���ⶨװ��B�й���������仯����ȷ���װ��A��ʣ�����������װ��C��������_________________________________��
��3����ֱ�Ӳ��װ��A��ʣ���������Ϊ19.2g����װ��B�й���Ӧ����_______g��
4��������A��ʣ�����19.2gΪFe��FexOy�Ļ��������м���������ϡH2SO4��ַ�Ӧ����H2 0.3g��
��1��������Fe����Ϊ________g��
��2��FexOy�Ļ�ѧʽΪ________��
���𰸡�4Fe(OH)2 +2H2O + O2 = 4Fe(OH)3 2 ����ʹ����Ʒ���汣�ָ����������Ʒ����Ϳ������� ����Ӵ��������ַ�Ӧ ���� 4FeS2+11O2![]() 2Fe2O3+8SO2 199.5 ���װ�õ������� �ž�װ�õĿ���������ը �����ɵ�CO2ȫ���ų���B���� ���Ҳ�����е�H2O��CO2��B���� 13.2 8.4 Fe O
2Fe2O3+8SO2 199.5 ���װ�õ������� �ž�װ�õĿ���������ը �����ɵ�CO2ȫ���ų���B���� ���Ҳ�����е�H2O��CO2��B���� 13.2 8.4 Fe O
��������
�⣺ 1����1������ˮ������������������������������ѧ����ʽΪ��2Fe+O2+2H2O�T2Fe��OH��2��
��2���������������ڿ����зֽ�������������ˮ��2Fe��OH��3=Fe2O3+2H2O����ת���ɽᾧˮʱ��ˮ���������ܱ�С������ת��������Fe2O32H2O����x��2��
2�������������������������ˮͬʱ�Ӵ�������Ҫ��ֹ����Ʒ���⣬����ʹ����Ʒ���汣�ָ��������Ʒ����Ϳ������ȴ�ʩ��
��������1��������Ӧ��Ӵ����Խ��ӦԽ���ң���������������Ӧ��ĽӴ�������ӿ췴Ӧ�ٶȣ�
��2�������������ˮ��Ӧ���������ᣬ����SO2ֱ���ŷſ��ܻ�������ꣻ
��3����FeS2��O2�ڸ��µ�����������Fe2O3��SO2����ѧ����ʽΪ��4FeS2+11O2![]() 2Fe2O3+8SO2��
2Fe2O3+8SO2��
��4�������ݷ�Ӧǰ����Ԫ�����������֪��FeS2��2SO2��2SO3��2H2SO4�����Ʊ�Ũ���������Ϊm����
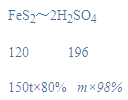
![]()
m=199.5t��
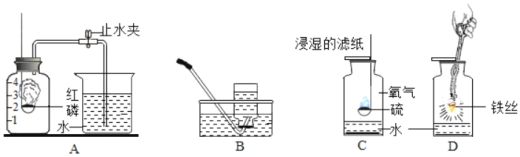
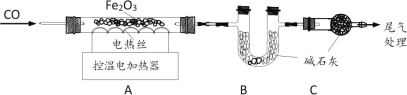
������1��������μӵķ�Ӧ��ʵ��ǰ��Ҫ����װ�õ������ԣ���������װ�ã��ȼ��װ�õ������ԣ���װ��ҩƷ��
2��CO���п�ȼ�ԣ��벣�����еĿ���������Ȼᷢ����ը������Ӧ��ͨCO�ž��������еĿ�����Ȼ���ټ��ȣ�
3����1������A���¶���700��������ȫ����ڣ�����ͨCO����ȴ�����Է�ֹ���ɵ�Fe�����������ܰ����ɵ�CO2ȫ���ų���B���գ�
��3����ʯ���ܹ�����ˮ�Ͷ�����̼������װ��C�������ǣ����տ����еĶ�����̼��ˮ������
��3�����װ��A��ʣ���������Ϊ19.2g������4.8g��������Ԫ�ص����������������ɶ�����̼����Ϊx��
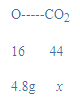
![]()
x=13.2g������װ��B�й���Ӧ����13.2g��
4�����������������Ϊy��
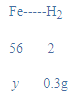
![]()
y=8.4g��
����FexOy������Ϊ��19.2g-8.4g=10.8g����Ԫ�ص�����Ϊ��10.8g-4.8g=6g������FexOy�Ļ�ѧʽΪFeO��

����Ŀ��������ij�о�С��̽��Ӱ�췴Ӧ���ʲ������ص����ʵ�����ݡ�
ʵ����� | ���������ܽ�Ũ��/% | �������� �ܽ�Һ��� | �¶�/�� | �������̵�����/g | �ռ��������mL | ��Ӧ���� ��ʱ�� |
�� | 5 | 1 | 20 | 0.1 | 4 | 16.75 |
�� | 15 | 1 | 20 | 0.1 | 4 | 6.04 |
�� | 30 | 5 | 35 | / | 2 | 49.21 |
�� | 30 | 5 | 55 | / | 2 | 10.76 |
��1��ͨ��ʵ���٢��Աȿ�֪����ѧ��Ӧ������_____�йأ�
��2��ͨ��ʵ��_____��������ʵ����ţ��Աȿ�֪����ѧ��Ӧ�������¶ȵĹ�ϵ��_____��
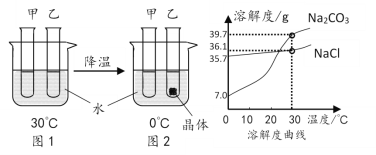
��3����һ����15%�Ĺ���������Һ��������Ϊ�˼�С��Ӧ���ʣ��ɼ�������ˮϡ�ͣ�����������������_____��ѡ���С���������䡱��������
��4��д���˷�Ӧ�����ֱ���ʽ_____��