��Ŀ����
����Ŀ����ѧʵ���ǽ��п�ѧ̽������Ҫ�ֶΡ�����д���пո�
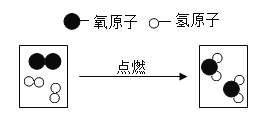
��1��������ǯ��ȡһС��ľ̿���ھƾ����ϼ�����ȼ�գ�Ȼ��ľ̿����ʢ�������ļ���ƿ�ڣ��۲�����
����ͼA��֪����ȼ��ȼ�յľ��ҳ̶���_____�йء�
��д���˷�Ӧ�ķ��ű���ʽ��_____��������Ӧ������_____
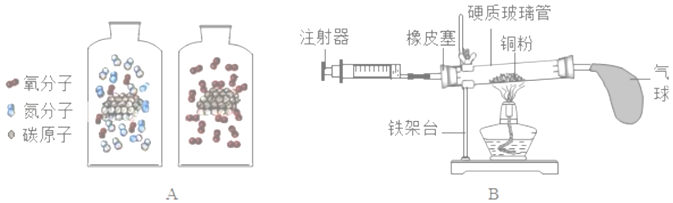
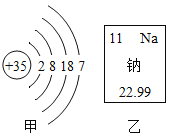
��2����ͼB��ʾ��װ�ÿɲⶨ�����������ĺ���
����ʵ���ԭ����ͨ��_____��ѡ���ѧ����������������������ȥ������е�һ�ֳɷ֣��Ӷ��ⶨ�������ij�ɷֵĺ�����
���ڲⶨ�У�ijͬѧ�õ����ƫС����д������һ��ԭ��_____��
����д����ʵ���з�����Ӧ�ķ��ű���ʽ��_____��
���𰸡���λ����������ӵĸ��� C+O2![]() CO2 ���Ϸ�Ӧ ��ѧ û�л����ƶ�ע�����Ļ���ʹ������ͭ�۳�ַ�Ӧ��װ��©���� Cu+O2
CO2 ���Ϸ�Ӧ ��ѧ û�л����ƶ�ע�����Ļ���ʹ������ͭ�۳�ַ�Ӧ��װ��©���� Cu+O2![]() CuO
CuO
��������
��1���پ�ͼA��֪����ȼ��ȼ�յľ��ҳ̶��뵥λ����������ӵĸ����йء�
����ͼʾ��֪��̼ȼ�������˶�����̼���˷�Ӧ�ķ��ű���ʽ��C+O2![]() CO2���÷�Ӧ���������һ�����ص㣬������Ӧ�����ǻ��Ϸ�Ӧ��
CO2���÷�Ӧ���������һ�����ص㣬������Ӧ�����ǻ��Ϸ�Ӧ��
��2��������ͭ�������ڼ���ʱ����������ͭ����ʵ���ԭ����ͨ����ѧ����������ȥ������е�һ�ֳɷ֣��Ӷ��ⶨ�������ij�ɷֵĺ�����
���ڲⶨ�У��õ����ƫС������ԭ������ǣ�û�л����ƶ�ע�����Ļ���ʹ������ͭ�۳�ַ�Ӧ��װ��©���ȡ�
�۸�ʵ����ͭ�������ڼ���ʱ����������ͭ��������Ӧ�ķ��ű���ʽ�ǣ�Cu+O2![]() CuO��
CuO��

 ��������һ���þ�ϵ�д�
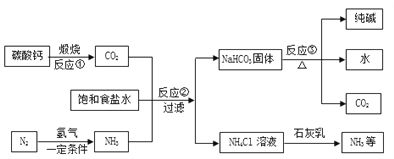
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
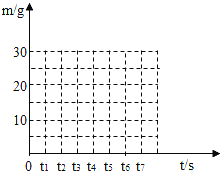
Сѧ��10����Ӧ����ϵ�д�����Ŀ��ͬѧ�Ǵ�ɽ�ϲɼ���һ��ʯ��ʯ������ȡ80 g����Ʒ��������ʵ�飨�������������չ����в������仯������÷�Ӧ������������m���뷴Ӧʱ�䣨t���Ĺ�ϵ�����
��Ӧʱ��t�Ms | t0 | t1 | t2 | t3 | t4 | t5 | t6 |
��Ӧ����������m�Mg | 80 | 75 | 70 | 66 | 62 | 58 | 58 |
��ش��������⣺
��1����ʯ��ʯ��ȫ��Ӧ������CO2��������
��2�����ʯ��ʯ��CaCO3������������д��������̣�
��3��������ͼ��ʾ������ͼ�У���������ʱ���������������m����ʱ�䣨t���仯�����ߣ�