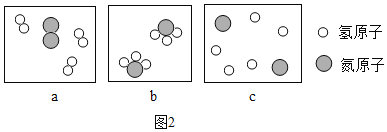
��Ŀ����
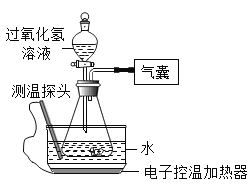
����Ŀ��ijС��ͬѧ��Ʋ�����ʵ�飬̽��Ӱ���������ֽ����ʵ����ء�
����������裩Ӱ���������ֽ����ʵ����ؿ������¶ȡ���Һ��Ũ�ȡ��������ࡣ
��ʵ���¼��ͬѧ�ǽ���6��ʵ�飬ʵ���¼���£�
ʵ����� | �� | �� | �� | �� | �� | �� |
����������Һ��Ũ�ȣ�%�� | 30 | 30 | 15 | 30 | 30 | 30 |
����������Һ�������mL�� | 6 | x | 6 | 6 | 6 | 6 |
ˮ����ˮ���¶� | 20 | 90 | 70 | 70 | 20 | 20 |
��ƿ�е����� | 0.5g NaCl | 0.5g FeCl3 | ||||
60���ڲ���������mL�� | 0.0 | 41.0 | 4.5 | 12.1 | 2.0 | 650.0 |
����������ۣ�
��1��ʵ�����Ӧ�������������Һ�����x��________mL��
��2��ʵ��١���ͨ���ⶨ��ͬʱ���ڲ���������������ȽϹ�������ķֽ����ʣ�������ͨ���ⶨ__________���ȽϹ�������ķֽ����ʡ�
��3���ó�������������Һ��Ũ��Խ��ֽ�����Խ�������������ݵ�ʵ����__________������ţ���
��4��ͨ��ʵ��١��ڡ��ܿɵó��Ľ�����_______________��
����˼�����ۣ�
��5��ͬѧ����Ϊʵ������������Ӧѡ��ʵ�����ѡʵ��ڵ�������____________��____________�������㣩��
��6������ΪӰ�����������Һ�ֽ����ʵ����ػ�����Щ�������ʵ����֤��IJ���
��������裩___________________��
��ʵ�������___________________��
��ʵ����ۣ�___________________��
���𰸡�6 ������ͬ��������������ʱ�� �ۢ� ����������ͬʱ���¶�Խ�ߣ���������ֽ�����Խ�� ��ͬʱ����ʵ��ڲ��������� ��Ҫ���ȣ��������ɣ� ����������Ӱ�����������Һ�ֽ����� ����ͬ�¶��£����ʢ��6mL 15%�Ĺ���������Һ����֧�Թ��зֱ����0.5g��1g�������̣�������ͬʱ���ڲ������������ ������1g�������̵��Թ��в����������������������������ͬ������£�������������Խ��������ֽ�����Խ��
��������
̽��Ӱ���������ֽ����ʵ����أ�
��������裺Ӱ���������ֽ����ʵ����ؿ������¶ȡ���Һ��Ũ�ȡ��������ࡣ
���ʵ��װ�á�6��ʵ�����ú�ʵ���¼����
��������ۣ�
��1��ʵ����м���Ĺ���������ҺӦ��������ʵ���м���Ĺ���������Һ�������ͬ����x��6mL��
��2��ʵ��١���ͨ���ⶨ��ͬʱ���ڲ���������������ȽϹ�������ķֽ����ʣ�������ͨ���ⶨ������ͬ��������������ʱ�����ȽϹ�������ķֽ����ʡ�
��3��Ҫ�ó�������������Һ��Ũ��Խ��ֽ�����Խ�����Ľ��ۣ���Ҫ��������������Һ��Ũ������Ϊ���������ж���ʵ�飬�������ݵ�ʵ���Ǣۢܡ�
��4��ʵ��١��ڡ��ܵı������¶ȣ����ͨ��ʵ��١��ڡ��ܿɵó��Ľ���������������ͬʱ���¶�Խ�ߣ���������ֽ�����Խ��
��˼�����ۣ�
��5��ͬѧ����Ϊʵ������������Ӧѡ��ʵ�����ѡʵ��ڵ���������ͬʱ����ʵ��ڲ��������٣���Ҫ���ȣ�ˮ���������ߵȡ�
��6������ʵ�����¶ȡ���Һ��Ũ�ȡ���������Ӱ�����������Һ�ֽ������⣬����������Ҳ����Ӱ�����������Һ�ֽ����ʣ����ʵ����֤���£�
������裺����������Ӱ�����������Һ�ֽ����ʡ�
ʵ�����������ͬ�¶��£����ʢ��6mL 15%�Ĺ���������Һ����֧�Թ��зֱ����0.5g��1g�������̣�������ͬʱ���ڲ��������������
ʵ����ۣ�������1g�������̵��Թ��в������������������������������ͬ������£�������������Խ��������ֽ�����Խ�졣

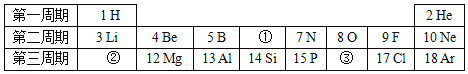
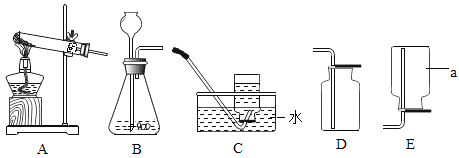
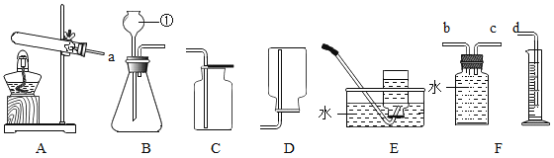
����Ŀ����ͼ��ʾ�ļס�������װ���У���ͷ�ι�������ij��Һ�壬ƽ����ƿ�г��루����룩��һ�����ʣ���ѹ��ͷ�ιܼ���Һ�壬һ��ʱ�����װ���е����������ʹ���Һ����������������Ӱ�죩����ιܺ���ƿ�������Լ�������
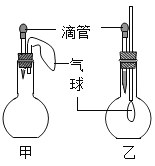
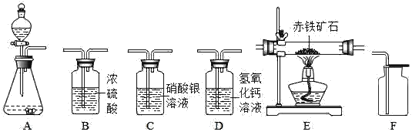
�� | �� | |
A | ϡ�����ͭƬ | ˮ�� CO2 |
B | ˫��ˮ��MnO2 | NaOH��Һ��CO2 |
C | Na2CO3��Һ��ϡ���� | NaOH��Һ��SO2 |
D | H2O��NH3 | ��������Һ��HCl |
A.AB.BC.CD.D