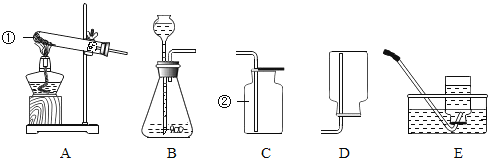
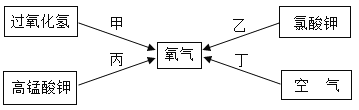
��Ŀ����
����Ŀ����Դ�ͻ���һֱ�ܵ����ǵĹ�ע��
��1��ú̿���ҹ���Դ�ṹ��ռ����Ҫ��λ������ʹ�����������ɳ�����չ��
�� ����ȼú������SO2�ŷţ��ܹ�����______��������γɡ���ú������һЩʯ��ʯ����ʹ�����Ķ�������ת��Ϊ����ƣ��÷�Ӧ�ķ���ʽ��_______��
�� ȼú������CO2����NH3��������Һ����CO2��Ӧ���Ʊ�����[CO(NH2)2]���ð�ˮ����CO2���Ƶ�̼泥�NH4HCO3�����õ���̼茶��ú�����٣��䷢����Ӧ�Ļ�ѧ����ʽΪ________��
��2����Ȼ����һ����Ҫ������ʯ�ͻ�����Դ����ش��������⣺
�� �������������ɵ�ȼ�ϵ��������ת����ʽ��Ҫ��__________��
a.��ѧ��ת��Ϊ���� b.����ת��Ϊ��ѧ�� c.��ѧ��ת��Ϊ����
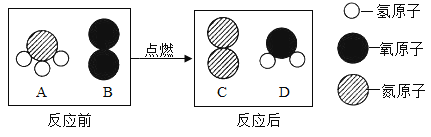
�� ��CH4��CO2��һ������������CO��H2ʱ��CH4��CO2�������������________���÷�Ӧ���ش�������________��
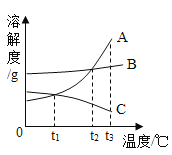
��3������������ֵ�ߡ���ȡ����ԭ����Դ�㡢ȼ�ղ�������Ⱦ���ص㣬����Ϊ����ɫ��Դ����������Դ���������»��������;�����ٵ��ˮ��������̫���ֽܷ�ˮ����ʹ�ø�Ч�����ֽ�ˮ�� ��ˮú������C��H2O(��)![]() CO��H2������Ȼ�����ѷ���CH4
CO��H2������Ȼ�����ѷ���CH4![]() C��H2�����н��з�չǰ;����________��
C��H2�����н��з�չǰ;����________��
���𰸡����� 2CaCO3+2SO2+O2=2CaSO4+2CO2 NH4HCO3=NH3![]() +CO2
+CO2![]() +H2O c 4:11 ���ٶ�����̼�ŷż�������ЧӦ �ڢ�
+H2O c 4:11 ���ٶ�����̼�ŷż�������ЧӦ �ڢ�
��������
(1) SO2������е�������ˮ������Ӧ�������ᣬ��ˣ��γɵ�����Ϊ�����ͣ�����д�����
ʯ��ʯ�е�̼������������������Ӧ��������ƺͶ�����̼�����ٶ���������Ⱦ������д��2CaCO3+2SO2+O2=2CaSO4+2CO2��
̼泥�NH4HCO3���ֽ����ˮ��������̼�Ͱ���������д��NH4HCO3=NH3![]() +CO2
+CO2![]() +H2O��
+H2O��
(2)�����������ڵ�ȼ�����·�Ӧ���ų�����������ѧ��ת��Ϊ�����ܣ�����д��c��
CH4��CO2��һ������������CO��H2����ѧ����ʽΪ��CH4+CO2 2CO+2H2��ÿ����16g�ļ��飬������44g�Ķ�����̼��CH4��CO2�������������16:44=4:11������д��4:11��
2CO+2H2��ÿ����16g�ļ��飬������44g�Ķ�����̼��CH4��CO2�������������16:44=4:11������д��4:11��
�÷�Ӧ���ش������Ǽ��ٶ�����̼�ŷż�������ЧӦ������д�����ٶ�����̼�ŷż�������ЧӦ��
�ٵ��ˮ��ʵ��װ�ú�ʵ��������ӣ�û�з�չǰ;���ʲ��������⣻������̫���ֽܷ�ˮ��������ԭ���ã���������Ⱦ���з�չǰ;���ʷ������⣻��ʹ�ø�Ч�����ֽ�ˮ�����Լ��ٷ�Ӧʱ�䣬ԭ���ã���������Ⱦ���з�չǰ;���ʷ������⣻��ˮú��������������һ����̼����Ⱦ������û�з�չǰ;���ʲ��������⣻����Ȼ�����ѷ�����Ӧ������ʵ��������ӣ�û�з�չǰ;���ʲ��������⣬����д���ڢۡ�

 ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
ѧ���쳵�����ּ��������ҵ�½����������ϵ�д� �����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д� Сѧ�����ҵ���ϴ�ѧ������ϵ�д�
Сѧ�����ҵ���ϴ�ѧ������ϵ�д� ���Ž�����ٰθ��νӹ㶫���������ϵ�д�
���Ž�����ٰθ��νӹ㶫���������ϵ�д�����Ŀ��ijʯ�ͻ����������ҵ�ʵ��Ա��15%������������Һϴ��һ����ʯ�Ͳ�Ʒ�еIJ������ᣬ���ʵ���������±���ʾ����һ����ʯ�Ͳ�Ʒ�в������������Ϊ100 g�������ò��������������������________________(д����ϸ�ļ������)��
ʵ����� | ����NaOH��Һ������ | ϴ�Ӻ���Һ��pH |
�� | 30 g | pH��7 |
�� | 40 g | pH��7 |
�� | 50 g | pH��7 |
����Ŀ��ijС��ͬѧ��Ʋ�����ʵ�飬̽��Ӱ���������ֽ����ʵ����ء�
����������裩Ӱ���������ֽ����ʵ����ؿ������¶ȡ���Һ��Ũ�ȡ��������ࡣ
��ʵ���¼��ͬѧ�ǽ���6��ʵ�飬ʵ���¼���£�
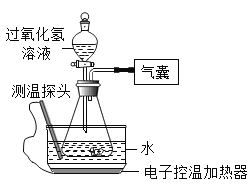
ʵ����� | �� | �� | �� | �� | �� | �� |
����������Һ��Ũ�ȣ�%�� | 30 | 30 | 15 | 30 | 30 | 30 |
����������Һ�������mL�� | 6 | x | 6 | 6 | 6 | 6 |
ˮ����ˮ���¶� | 20 | 90 | 70 | 70 | 20 | 20 |
��ƿ�е����� | 0.5g NaCl | 0.5g FeCl3 | ||||
60���ڲ���������mL�� | 0.0 | 41.0 | 4.5 | 12.1 | 2.0 | 650.0 |
����������ۣ�
��1��ʵ�����Ӧ�������������Һ�����x��________mL��
��2��ʵ��١���ͨ���ⶨ��ͬʱ���ڲ���������������ȽϹ�������ķֽ����ʣ�������ͨ���ⶨ__________���ȽϹ�������ķֽ����ʡ�
��3���ó�������������Һ��Ũ��Խ��ֽ�����Խ�������������ݵ�ʵ����__________������ţ���
��4��ͨ��ʵ��١��ڡ��ܿɵó��Ľ�����_______________��
����˼�����ۣ�
��5��ͬѧ����Ϊʵ������������Ӧѡ��ʵ�����ѡʵ��ڵ�������____________��____________�������㣩��
��6������ΪӰ�����������Һ�ֽ����ʵ����ػ�����Щ�������ʵ����֤��IJ���
��������裩___________________��
��ʵ�������___________________��
��ʵ����ۣ�___________________��