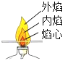
��Ŀ����
����Ŀ��ijʳƷ�İ�װ���з���һС����������������ѧ��ȤС���ͬѧ�ԡ����������ijɷֲ����˺��棬���ǽ�һ������������������ֽ�ϣ�������������������һЩ�Һ�ɫ��ĩ��һЩ��ɫ��ĩ��Ϊ�ˣ�չ������̽�����
��������⣩�����������лҺ�ɫ�ķ�ĩ�ͺ�ɫ�ķ�ĩ�ֱ���ʲô��
���������ϣ�ʳƷ������Ҫ����ΪʳƷ�ױ������е�������ˮ������������������ʣ�ʹ�á������������ӳ�ʳƷ�ı����ڣ�����������ϡ���ᷴӦ�����Ȼ�����ˮ������Һ����������Ӷ��ʻ�ɫ��
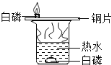
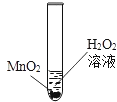
��������룩С���������������лҺ�ɫ�ķ�ĩ������ͭ����ɫ�ķ�ĩ��ͭ��
С�죺�����������лҺ�ɫ�ķ�ĩ������ͭ��̼�ۣ���ɫ�ķ�ĩ��ͭ��
С���������������лҺ�ɫ�ķ�ĩ�����ۺ�̼�ۣ���ɫ�ķ�ĩ����������
�����۷�����ͨ�����ۣ�ͬѧ��һ����ΪС���IJ�������ȷ�ġ������ǣ�_______________________��
��ʵ��̽��������������ǵ�̽��������ʵ�����ݲ���������
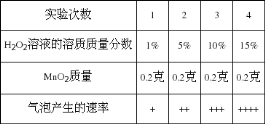
ʵ�鲽�輰���� | ʵ������ | ʵ����� |
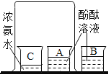
�ô����ӽ���ֽ�ϵġ���������������������� | ���������� �˲��ֺ�ɫ���� | ___________________ |
�� ȡ����ʣ��ķ�ĩ���Թ��У�����������_________���۲����� | _________________________________ | С�����������д����ѧ ����ʽ�� _________________________ |
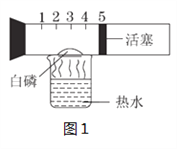
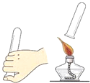
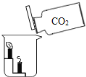
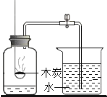
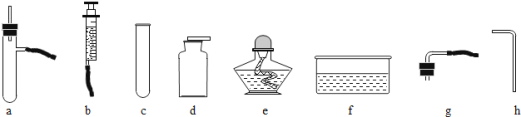
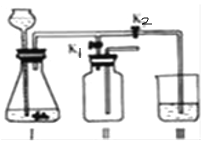
����չ̽����Ϊ�˽�һ��̽�����������������ʣ���ȤС���ͬѧ��ȡһ��������������ĩװ��Ӳ�ʲ������У���������ͼ��ʾ��ʵ�顣
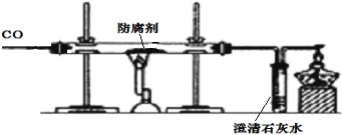
(1)��ʵ����ֳ�CO���еĻ�ѧ������___________________________����ʵ�ʲ���������������ֵ���ԭ����____________________________________________��
(2)ʵ�������ͨ��������Ӧǰ��Ӳ�ʲ������й������ʵ����������Ƿ��ַ�Ӧ���ɫ�������ʱ�ɺ�ɫ���ҵ�������С��������С��ԭ��______________________��
���𰸡�������ֻ�����ڳ�������ͬʱ��ˮ������������Ӧ ��ɫ������������ ϡ��� ��ɫ��ĩ��ʧ����Һ����ɫ���ɫ���Թܵײ��к�ɫ��ĩ�� Fe2O3+6HCl=2FeCl3+3H2O ��ԭ�ԺͿ�ȼ�ԣ� ��Ӧǰ��Ҫͨ��һ����̼����������������ֵ ����������Ԫ�ر����ߡ�
��������
(1)ʹ�÷�������Ŀ�ľ������÷�������ʳƷ��װ�ڵ�������ˮ��Ӧ��Ϊ���ڵ�ʳƷ�ṩһ�����������Ļ������Ӷ��ӳ�ʳƷ�ı����ڡ���λͬѧ�IJ������漰��������ֻ�����ڳ�������ͬʱ��ˮ������������Ӧ�����������ֻ�����ڳ�������ͬʱ��ˮ������������Ӧ����2������������ֻ�������ܱ����������������ɫ����������������3��ʵ������֤�����Ĵ��ڲ���ȥ������������С������ȷ��ʣ������п϶�������������֤�������Ĵ��ڿ�������������ϡ���ᷴӦʹ��Һ���ɫ˵���������Ĵ��ڣ�����ϡ��������4�������������ᷴӦ���ɵ��Ȼ�������ˮ����Һ�ʻ�ɫ��̼�۲������ᷴӦ�Ҳ�����ˮ�������ɫ��ĩ��ʧ����Һ����ɫ���ɫ���Թܵײ��к�ɫ��ĩ����5��ʵ��������������ϡ���ᷴӦ�����Ȼ�����ˮ���ʻ�ѧ����ʽдΪFe2O3+6HCl=2FeCl3+3H2O����6��CO��������е���������Ӧʱ����ȡ�������е���Ԫ�ؽ���������ԭΪ��������CO�Ļ�ԭ�ԣ�β���е�CO�þƾ��Ƶ�ȼ��ȼ�մ�������CO�Ŀ�ȼ�ԣ����ԭ�ԺͿ�ȼ�ԡ���7����Ӧǰ��Ҫ��ǰͨ��CO�ž����ڿ�������ը����������ͨ��CO�����������CO��ʵ��������������ֵ�����Ӧǰ��Ҫͨ��һ����̼����������������ֵ����8����Ӧ��CO��ȡ���������е���Ԫ�ر�������CO2��ʹ�������ڹ�����������٣���������������Ԫ�ر�������

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�