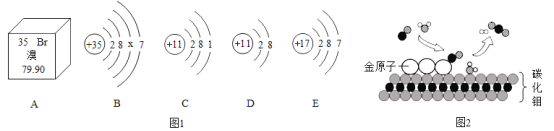
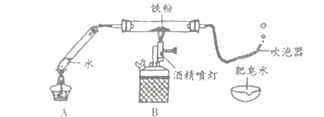
��Ŀ����
����Ŀ�����������ʵ��̽����
��ʵ��̽����
ʵ������ | ��¼���� |
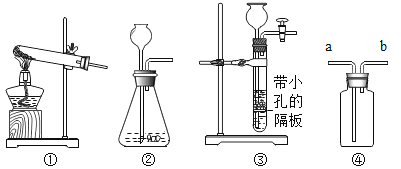
A.�������ʹ����豸̽�����ס������ڿ� ����ȼ��ʱ�ĺ������
| ȼ�պ��ף�ƿ��ʣ��������������Ϊ8.85% ȼ�հ��ף�ƿ��ʣ���������������Ϊ3.21% |
B.��������ԭ���ⶨ�������������������
| ��Ӧǰ�ⶨ�����ƿ�ݻ����۳�ҩƷ�����ܺ�ƿ����ռ�ݻ���Ϊ125.0mL ��Ӧ��ⶨ��������ƿ�е����Ϊ25.0mL |
��1�����Ͱ���������ͬ����Ԫ����ɵIJ�ͬ___________��������������������������������Aʵ��ʱ��ȼ�շ�Ӧ�Ļ�ѧ����ʽ�ɱ�ʾΪ___________��
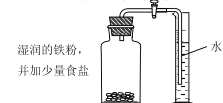
��2��ʵ��A�IJⶨ������ڲ�������װ�ò�©�������ס�����������ϴ����Ҫԭ��___________��
��3��ʵ��B�У���������Ϊ�ӿ�ʵ����̶���ȡ�Ĵ�ʩ��___________��___________�������ֱ�ţ���
���ڼ���ƿ�н��� ������ƿ����©�� ��ʹ�����۶���ʹ����˿ �ܼ�����ʳ��
��4���������ʵ��������_________��д��ѧʽ�������˻�ѧ��Ӧ�����ݱ��м�¼���ݣ��ɼ�����������������������Ϊ__________��
����˼����չ��
ͭ�ڳ�ʪ�Ŀ���������ͭ�̻�ѧʽΪ[Cu2(OH)2CO3]ʱҲ�ܺ������ӻ�ѧʽ���Կ�����ͭ�̵�������ͭ��ˮ�������е�___________������Ӧ�Ķ����̣����ԣ�ʵ��B_____ (�� ����������������)��ͭ�۴������ۡ�
���𰸡����� ![]() �����Ż��Ⱥ��ͣ�������ȼ�� �� �� O2��H2O 20% ������������̼ ����
�����Ż��Ⱥ��ͣ�������ȼ�� �� �� O2��H2O 20% ������������̼ ����
��������
[ʵ��̽��]
��1�����Ͱ���������ͬ����Ԫ����ɵIJ�ͬ���ʣ��ڵ�ȼ�������£�����������Ӧ�������������ף�
��2���ò�����Ũ�ȵĵ��Ӵ�������÷�Ӧ��װ��������Ũ�ȷֱ�Ϊ8.85%��3.21%����ȼ�������ĽǶȷ��������������������Ҫԭ���ǣ������Ż��Ⱥ��ͣ�������ȼ�գ�
��3������ʵ���У����۱���˿�������ĽӴ��������Ӧ���ʻ�ӿ죻�Ȼ����ܼӿ����۵���ʴ���ʣ�
��4���������ʵ��������������ˮ���������˻�ѧ��Ӧ�������ˮ�������Ϊ���ĵ���������������ݱ��м�¼���ݿɼ�����������������������Ϊ��![]() ��
��
[��˼����չ]
��ѧʽ���Կ�����ͭ�̵�������ͭ��ˮ�������е�������������̼������Ӧ�Ĺ��̣�ͭ����ʴ��Ҫ������̼���������ж�����̼�ĺ�����0.03%��ͭ��ˮ��������������̼��Ӧʱ�����ڶ�����̼�����ͣ�ͭ���ܰ�װ���е������ľ������Բ�����ͭ�۴������۲ⶨ�����������ĺ�����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��t1��ʱ�����ס��ҡ������ֹ����20�ˣ��ֱ����ʢ��50��ˮ���ձ��У�����ܽ���������±�
���� | �� | �� | �� |
δ�ܽ�Ĺ���/g | 5.4 | 0 | 2 |
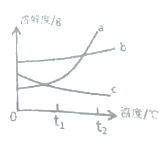
��1��һ���DZ�����Һ����_______��t1��ʱ�����ܽ��Ϊ_______��
��2��ͼ�б�ʾ�����������________��
��3��t1��ʱ�����ұ����ܽ�ȴ�С��ϵΪ_______��
��4��t2��ʱ��ȡ�������ļ��ұ��ı�����Һ���ֱ����������������ˮ���������������Ĵ�С��ϵΪ______���ü��ұ���ʾ��