��Ŀ����
����Ŀ��ij��ѧ������ʦ��ָ����̽������ˮ�����ķ�Ӧ��
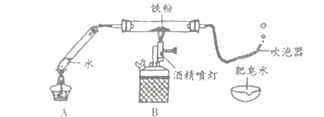
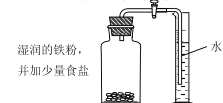
��1������ͼ��ʾװ��ҩƷ������װ�ã��г���������ȥ��������Aװ�õ�������______��
��2������һ��ʱ���Bװ���еĻ�ɫ������ڣ������������������ݣ����������Ϸ�����ȼ�ŵ�ľ���������ݣ��ܲ�������������������______��
��3��ͬѧ�����ۺ���Ϊ������ˮ������Ӧ���ɵĹ�����������һ�������������������ڵĺ�ɫ�����л����ܺ�����ʣ���������
���������ϣ����������������֣�![]() ������
������![]() �Ǻ���ɫ�ģ���
�Ǻ���ɫ�ģ���![]() �Ӵ����������ɺ�ɫ��Ϊ����ɫ��
�Ӵ����������ɺ�ɫ��Ϊ����ɫ��
��ʵ��̽����
�ٽ����еĺ�ɫ���嵹����ƽ���ڰ�ֽ�ϣ�û�з��ֺ���ɫ���ʣ�˵�����ɵĹ��岻������______��һ���֮��ɫ���岻��ɫ�����ɫ������һ��û��______�������������ƣ�
��ȡ������ɫ������������������![]() ��Һ�����ֺ�ɫ���岿���ܽ⣬����______ɫ�������ʳ��֣�˵����ɫ������һ��������������������
��Һ�����ֺ�ɫ���岿���ܽ⣬����______ɫ�������ʳ��֣�˵����ɫ������һ��������������������
��̽�����ۣ�����ˮ���������û���Ӧ�����ɵĺ�ɫ������![]() ���÷�Ӧ�Ļ�ѧ����ʽΪ_______��
���÷�Ӧ�Ļ�ѧ����ʽΪ_______��
���𰸡��ṩˮ���� ��������![]() ��
�� ![]() ������������ �������� ��
������������ �������� �� ![]()
��������
��1��Aװ���ڼ��ȵ������²���ˮ��������Aװ�õ������ǣ��ṩˮ������
��2����ȼ�ŵ�ľ���������ݣ��ܲ���������˵�������е�������������
[ʵ��̽��]�������������Ǻ���ɫ�ģ��������������к�ɫ���壬ƽ���ڰ�ֽ�ϣ�û�з��ֺ���ɫ���ʣ�˵�����ɵĹ��岻������������������FeO�Ӵ����������ɺ�ɫ��Ϊ����ɫ����һ���֮��ɫ���岻��ɫ�����ɫ������һ��û������������
�������û���CuSO4��Һ��ͭ����������������CuSO4��Һ��Ӧ����ȡ������ɫ����������������CuSO4����Һ�����ֺ�ɫ���岿���ܽ⣬���к�ɫ�������ʳ��֣�˵����ɫ������һ��������������������
[̽������] ����ˮ���������û���Ӧ�����ɵĺ�ɫ������Fe3O4���÷�Ӧ�Ļ�ѧ����ʽΪ��3Fe+4H2O![]() Fe3O4+4H2��
Fe3O4+4H2��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ����1����ͬʵ��Է�Ӧ�����в�ͬҪ��ijͬѧ̽��CO2��ȡʵ�飬��Ҫ����⡣ ��ѡ���Ʒ�Ӧ���ʵķ������ٷ�Ӧ���Ũ�� �ڷ�Ӧ���״̬
��ѡ�Լ���A.ϡ���� B.Ũ���� C.��״ʯ��ʯ D.̼������Һ E.��ĩ״ʯ��ʯ
��д�±����ش��������
ʵ������ | �������ʵķ��� | ��ѡ�õ�����Լ� |
�������Ӧԭ�� | �� | ___________��D |
CO2��ʵ�����Ʒ� | ___________�� | A��C |
д��ʵ�����Ʊ�CO2�Ļ�ѧ��Ӧ����ʽ��______________________��
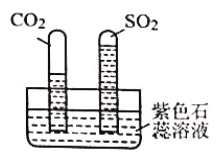
��2����֪��CO2��SO2���������ԣ����в����ԡ�
ʵ�飺ˮ���е�����ɫʯ����Һ�����ռ�������������Թ�ͬʱ������ˮ���У�Ƭ�̺�ʵ����������ͼ��ʾ��˵����ͬ�����µ��ܽ��ԣ�SO2 ___________���>����<����CO2���Թ�����Һ���___________ɫ��
����Ŀ�����������ʵ��̽����
��ʵ��̽����
ʵ������ | ��¼���� |
A.�������ʹ����豸̽�����ס������ڿ� ����ȼ��ʱ�ĺ������
| ȼ�պ��ף�ƿ��ʣ��������������Ϊ8.85% ȼ�հ��ף�ƿ��ʣ���������������Ϊ3.21% |
B.��������ԭ���ⶨ�������������������
| ��Ӧǰ�ⶨ�����ƿ�ݻ����۳�ҩƷ�����ܺ�ƿ����ռ�ݻ���Ϊ125.0mL ��Ӧ��ⶨ��������ƿ�е����Ϊ25.0mL |
��1�����Ͱ���������ͬ����Ԫ����ɵIJ�ͬ___________��������������������������������Aʵ��ʱ��ȼ�շ�Ӧ�Ļ�ѧ����ʽ�ɱ�ʾΪ___________��
��2��ʵ��A�IJⶨ������ڲ�������װ�ò�©�������ס�����������ϴ����Ҫԭ��___________��
��3��ʵ��B�У���������Ϊ�ӿ�ʵ����̶���ȡ�Ĵ�ʩ��___________��___________�������ֱ�ţ���
���ڼ���ƿ�н��� ������ƿ����©�� ��ʹ�����۶���ʹ����˿ �ܼ�����ʳ��
��4���������ʵ��������_________��д��ѧʽ�������˻�ѧ��Ӧ�����ݱ��м�¼���ݣ��ɼ�����������������������Ϊ__________��
����˼����չ��
ͭ�ڳ�ʪ�Ŀ���������ͭ�̻�ѧʽΪ[Cu2(OH)2CO3]ʱҲ�ܺ������ӻ�ѧʽ���Կ�����ͭ�̵�������ͭ��ˮ�������е�___________������Ӧ�Ķ����̣����ԣ�ʵ��B_____ (�� ����������������)��ͭ�۴������ۡ�
����Ŀ�����������������У�ѡ�������ȡ������̼��װ�ã����ж�����̼����ȡ������̽����

(1)ָ��ͼ�����������ƣ���_____________��
(2)��ȡ���ռ�������̼��ѡ����ͼ�����е�_____________(�����)����Ӧ�Ļ�ѧ����ʽΪ__________________________��
(3)�ռ���һƿ������̼���������̼�Ƿ��ռ����ķ�����__________________________��
(4)Ϊ��̽��������̼����ˮ������Ӧ���ɾ������Ե����ʣ��ס�����ͬѧ�ֱ������̽��ʵ��ķ���(��ͼ�ס�����ʾ)����ʵ�顣ͼ�Ҳ��õ��Ķ仨Ϊ��ʯ����ҺȾ����ɫ�ĸ����ֽ����

��������ס���ͬѧһ�����̽����
������ | (��) | (��) | (��) | (��) |
���� | ���ɫ | ����ɫ | ����ɫ | ���ɫ |
����ͼ�ס�����CO2ʹʯ����Һ���ɫ��ԭ��_______________��
������Ϊ�ס�����λͬѧ��ʵ��̽��������������˵��������ɣ�_______________________��