��Ŀ����
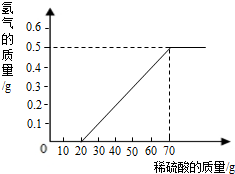
��һ��ʯ��ʯ��Ʒ�ijɷ���CaCO3��SiO2������С��ͬѧ��100g�����5�μ��뵽35g��ʯ��ʯ��Ʒ�У���֪SiO2�������ᷴӦ�����õ����²������ݺ�ͼ��
| ���� | ��1�� | ��2�� | ��3�� |
| �������������/g | 20 | 20 | 20 |
| ʣ����������/g | 30 | a | 20 |
����㣺
��1����2�μ��������aΪ g��
��2��ʯ��ʯ��Ʒ��̼��Ƶ���������������������ȷ��0��1%��
��3��10%��CaCl2��Һ����·�汣ʪ����������5��ʵ������Һ���10%��CaCl2��Һ�����������Һ�м���������ʯ��ʯ��ĩ����ȫ��Ӧ����ˣ���ʱ����Ҫ����Һ�м���ˮ���ٿˣ�������ʵ���������Һ��ʧ���Բ��ƣ�����������ȷ��0��1g��
��1��25��2��57��1% ��3��163��5g
���������������1��û�м�����ǰ�����������Ϊ35g����һ�μ���������������������5g������Ӧ��5g̼��ƣ���ô��2�μ���������ֻᷴӦ5g̼��ƣ������������Ϊ25g����aΪ25��
��2������ͼ���������4�μ����������Ʒ��̼�����ȫ��Ӧ����ʱʣ��Ĺ���SiO2����Ϊ15g����̼��Ƶ�����Ϊ35g-15g=20g,��ʯ��ʯ��Ʒ��̼��Ƶ���������=20g/35g��100%=57��1%
��3����ͼ����֪��ÿ����20g���ᣬ������5g CaCO3����100g��������25g CaCO3��
��100g������ȫ��Ӧ����Һ��CaCl2������Ϊx������CO2������Ϊy
CaCO3 + 2HCl === CaCl2 + H2O + CO2��
100 111 44
25g x y
100/111 = 2g5/x x = 27��75g
100/44 = 25g/y y=11g
����CaCl2��Һ������Ϊ25g+100g-11g=114g
�軹��Ҫ��ˮ������Ϊz
27��75g/(114g+z) ��100%=10%
z=163��5g
�𣺣�1��aΪ25g��
��2��ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ57��1%��
��3������Ҫ����Һ�м���163��5gˮ��
���㣺���û�ѧ����ʽ�ļ���

 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�