��Ŀ����
����Ŀ���Ķ�������ն��ġ�
�����Ǽ�ˮ����С������֮��ĵ��Ĵ���ʳ��������ۡ������ʡ�ά����C�ȶ���Ӫ�����ʣ��Dz����ϵ���ζʳ�ġ�

��1����������ҪӪ�����ʵĺ�����ÿ100g��
������/g | ֬��/g | ����/g | ��/mg | ��/mg | ά����C/mg |
1.5��2.3 | 0.4��0.94 | 17.5��28.0 | 11��60 | 15��68 | 20��40 |
�����п������ֱ䣬��ˮ�����ܷ�ֹ�ֱ䣬��������Ӫ��������ʧ��������Ƭ����ʵ�飬�����ʳ��Ʒ�ʡ����ۺ�����ά����C���������ʱ��ı仯����:

�������̡���ѿʱ�������غ����������ߣ��������ض�ʳ�ɵ����ж��������ǰѱ��̡���ѿ�IJ�λ������Ҳ����ʳ�á���ʳ���⣬������������Ϊԭ�Ϲ㷺����ҽҩ����������֯����ֽ�ȹ�ҵ�С�
�����������ݻش��������⡣
��1����1�еġ��ơ�ָ����________���Ԫ�ء���ԭ�ӡ�����
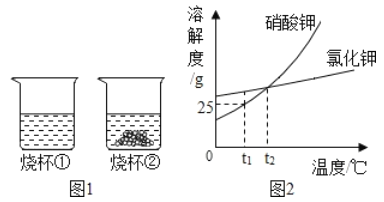
��2����ͼ1��֪��60min�ڣ�______������ţ���
A����30min��10min���Է�ֵ��
B����10min��Ӳ�ȡ�ҧ��о������Ա仯
C���Ž���ʱ������ӣ�ճ���ԡ����Է�ֵ������
��3����ͼ2��֪������Ƭ�ĵ��ۺ��������ʱ��Ĺ�ϵ��________________��
��4�����̡���ѿ����������ʳ�ã���ԭ����___________________��
��5������˵������ȷ����____������ţ���
A�������Ĵ���ʳ����֮һ B������ά����C�������
C��ˮ�ݿɷ�ֹ����Ƭ�։� D��������Ӧ�õ�ǰ������
���𰸡�Ԫ�� ABC ������������ͬʱ�����о���Χ�ڣ�����Ƭ�ĵ��ۺ��������ʱ����ӳ������� �������̡���ѿʱ�������غ����������ߣ��������ض�ʳ�ɵ����ж� B
��������
��1�����ʶ�����Ԫ����ɵģ����Ա�1�еġ��ơ�ָ����Ԫ�أ�
��2��A����ͼ1�е���Ϣ��֪������30min��10min���Է�ֵ�ߣ���A��ȷ��
B����ͼ1�е���Ϣ��֪������10min��Ӳ�ȡ�ҧ��о������Ա仯����B��ȷ��
C����ͼ1�е���Ϣ��֪�����Ž���ʱ������ӣ�ճ���ԡ����Է�ֵ�����ͣ���C��ȷ��
��3����ͼ2�ṩ����Ϣ��֪��������������ͬʱ�����о���Χ�ڣ�����Ƭ�ĵ��ۺ������Ž���ʱ����ӳ������ͣ�
��4��������ṩ����Ϣ��֪���������̡���ѿʱ�������غ����������ߣ��������ض�ʳ�ɵ����ж���
��5��A������ɡ������Ǽ�ˮ����С������֮��ĵ��Ĵ���ʳ�����֪��A��ȷ��
B���ɱ�1�ṩ����Ϣ��֪�����۵ĺ�����ߣ���B����
C������ɡ������п������ֱ䣬��ˮ�����ܷ�ֹ�ֱ䣬��������Ӫ��������ʧ������֪��C��ȷ��
D������ɡ���ʳ���⣬������������Ϊԭ�Ϲ㷺����ҽҩ����������֯����ֽ�ȹ�ҵ�С�����֪D��ȷ��
�ʴ�Ϊ��
��1��Ԫ�أ�
��2��ABC��
��3��������������ͬʱ�����о���Χ�ڣ�����Ƭ�ĵ��ۺ������Ž���ʱ����ӳ������ͣ�
��4���������̡���ѿʱ�������غ����������ߣ��������ض�ʳ�ɵ����ж���
��5��B��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��Ϊ�õ��ߴ���̼��ƣ�ʵ������Na2CO3��Һ��CaCl2��Һ��Ӧ��ȡ���ֽ�150gCaCl2��Һ���Ĵμ���ʢ��100gNa2CO3��Һ���ձ��У���ַ�Ӧ���Ĵβ����������ݼ��±���
���� | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
�ۼƼ���CaCl2��Һ������/g | 40 | 80 | 120 | 150 |
�ձ�����Һ��������/g | 134 | 168 | 202 | 232 |
��1����___________��ǡ����ȫ��Ӧ����ʱ���ɳ�����������Ϊ___________g��
��2�����Ĵβ���ʱ��������Һ�е�������___________���ѧʽ����
��3��CaCl2��Һ�����ʵ���������Ϊ___________����д��������̣�