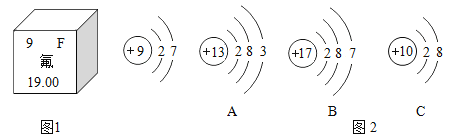
��Ŀ����
����Ŀ���⻯��(KI)���治������ʡ�ʵ��С���������ʵ��̽��KI���ʵ����ء�
����������� KI���ʵ�������ʲô��
���������ϡ�
�� KIΪ��ɫ��ĩ����¶�ڿ����л���ûᱻ����Ϊ��(I2)�����Ʊ��ʡ�
�� ��ˮ�к��϶�KIʱ���μӵ�����Һ����ɫ������ɫ
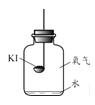
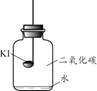
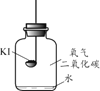
������ʵ�顿�ֱ�ȡ����KI��ȼ�ճ��У��ٷֱ����ʢ�в�ͬ���ʵļ���ƿ�У������������������۲졣
ʵ��1 | ʵ��2 | ʵ��3 | ʵ��4 |
|
|
|
|
����䳱��������� | �������������� | ����䳱���������������� | ����䳱�������� |
����ʵ�飺ȡʵ��1�������ƹ����ܽ⣬���������Һ����Һ����ɫ��
ȡʵ��4��������ɫ�����ܽ⣬���������Һ����Һ����ɫ��
����������ۡ�
(1)ʵ��3��Ŀ����_______��
(2)�Ա�ʵ��_______�����Եó�KI����һ����ˮ�йء�
(3)������ʵ�����֪��KI���ʵ�������_______��
���������2��CO2��������ʲô��
������ʵ�顿�ֱ�ȡ10 mLͬŨ�ȵ�KI��Һ��3֧�Թ��У������Թ�2��ͨ��CO2�����Թ�3�еμӼ�������ֱ����Һ��pH�������Ӻ۲���Һ����ɫ�������Թ��е��������Һ���۲���Һ����ɫ��ʵ�������¼���£�
�Թ���� | 1 | 2 | 3 |
��ҺpH | pH=8.5 | pH=6.5 | pH=4.5 |
��Һ��ɫ | ��ɫ | dz��ɫ | ��ɫ |
�μӵ�����Һ�����ɫ | ��ɫ | ��ɫ | ����ɫ |
�ϳ�ʱ��۲쵽�Թ�1����Һ��Ϊ��ɫ��
����������ۡ�
(4)�Թ�1ʵ���Ŀ����_______��
(5)CO2��KI���ʹ����е�������_______��
����˼�����ۡ�
(6)̽��KI��������ʱ��ͬѧ���ų��˵�����ϡ�������Ӱ�죬��ԭ����______��
���𰸡� ֤���� H2O�� CO2 ��O2�������£��⻯���Ƿ���� 2��4 H2O ��O2ͬʱ���� ���Թ�2��3���Ա� �ṩ���Ի������ӿ�⻯�ر��� ������ϡ�����廯ѧ�����ȶ�
��������(1)ʵ��3��KI���� H2O�� CO2 ��O2�������£����ֱ䳱����������������Ŀ������ H2O�� CO2 ��O2�������£��⻯���Ƿ���ʣ�(2)�����ĸ�ʵ������ʵ��2��ʵ��4��֪KI����һ����ˮ�йأ�(3) �����ĸ�ʵ��ɵã�KI���ʵ�������H2O ��O2ͬʱ���ڣ�(4) �Թ�1 ���ṩ���Ի��������Թ�2��3�������ԣ������Թ�1�Ǻ��Թ�2��3���Աȣ�(5)������̼��ˮ����̼�ᣬ̼�������ԣ��ɼӿ�⻯�ر��ʵ����ʣ�(6)������ϡ�����廯ѧ�����ȶ�����KI���ʼ���û��Ӱ�졣

����Ŀ��С��������е�ˮ������ˮʱ�����ֺ�����Щ��ɫ�Ĺ��塣Ϊ��̽����ɷ֣�����ȤС��ͬѧ�������Ϻ��֪��Щ��ɫ�Ĺ�����ˮ��������������Ϊˮ�к���Ca(HCO3)2��Mg(HCO3)2�ȿ����������ڼ���ʱ����CaCO3��Mg(OH)2��
��1�����н϶�Ca(HCO3)2��ˮ��_____������ʱ������Ӧ�Ļ�ѧ����ʽΪ_____��
��2��С����Ϊ��ˮ���ijɷ�ֻ��CaCO3����С�ײ�ͬ�⣬��ΪҲ����ֻ��Mg(OH)2����������_____��
��3��С��Ϊ��֤���Լ��IJ��룬�������ʵ�鷽����
ʵ����� | �֡����� | �ᡡ���� |
��ȡ����ˮ�����Թ��У����������_____�� | �۲쵽����_____�� | ˮ������CaCO3 |
����ٷ�Ӧ����Թ��еμ�2-3��NaOH��Һ | �۲쵽����_____�� | ˮ����û��Mg(OH)2���ҵIJ������ |
��3��С�ײ��Ͽ�С���Ľ��ۣ���Ϊ����ʵ�������ȱ�ݣ�ԭ����_____��
����Ŀ�����з������ܴﵽ����Ŀ�ĵ��ǣ� ��
ѡ�� | ���ʣ�������Ϊ���ʣ� | ���� |
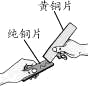
A | N2��O2�� | ���������ͨ������ͭ�� |
B | Fe�ۣ�Cu�ۣ� | ��������ϡH2SO4��ַ�Ӧ�����ˣ�ϴ�ӣ����� |
C | CaO��CaCO3�� | �������� |
D | FeCl2��Һ��CuCl2�� | ��������Fe�ۣ���ַ�Ӧ����� |
A.AB.BC.CD.D
����Ŀ��ij��ѧ��ȤС���ͬѧ��ͨ����ѯ��ʦ��������������Һ��Ũ���ᷴӦ���Ʊ�һ������SO2��Na2SO3+H2SO4��Ũ��=Na2SO4+SO2��+H 2O������ʦ�������ṩ��һƿ���õ�����������Һ����ȤС���ͬѧ�ֳɼס�����С��Ը�ƿ����������Һ�ɷֽ���ʵ��̽����
���������ϣ�
�� Na2SO3���ȶ����ڿ������ױ�����������Na2SO4��
��SO32-��SO42-������Ba2+��Ӧ������ɫ������
��BaSO3 ��������������ϡ���ᷴӦ���䷴Ӧԭ���� BaCO3��̼������ϡ���ᷴӦ���ơ�
(1)��������⣩�ٸ�ƿ��Һ�����ʵijɷ���ʲô��
�ڸ�ƿ��Һ���������Ƶ����������Ƕ��٣�
���������⣩ Na2SO3���ʵ�ԭ���ǣ�_______________________�����û�ѧ����ʽ��ʾ��
(2) ���������룩
����1��û�б��ʣ��ɷ���Na2SO3��
���� 2����ȫ���ʣ��ɷ���Na2SO4��
����3��_____________��
��ʵ��̽�������ס�������ֱ����ʵ��̽����Һ�Ƿ���ʣ�
С�� | ʵ����� | ���� | ���� |
���� | ȡ������Ʒ���Թ��м������ϡ��� | �������� | û�б��ʣ�����Na2SO3 |
���� | ȡ������Ʒ���Թ��м����Ȼ�����Һ���ټ�������ϡ���ᡣ | ������ɫ������ ________ | �Ѳ��ֱ��� |
����ͬѧʵ���з����Ļ�ѧ����ʽΪ��________________________����ͬѧ���ɼ��鷽����������������_________________��
(3) ��ʵ��̽�����������������ʵ��װ�òⶨNa2SO3��Һ��������������������![]() ��
��

��Ҫ�ⶨNa2SO3��Һ����������������ʵ������Ҫ��õ�����������������Һ��Ʒ�������Լ�_______������
��Dװ�õ�����Ϊ______________________________��