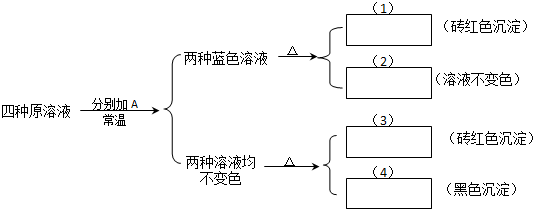
��Ŀ����
��1��25��ʱ��pH=12��KOH��Һ��pH=12��K2CO3��Һ�У���ˮ�������C��OH-��֮���� ��
��2����֪������0.1mol?L-1 CH3COONa��ҺPHԼΪ8
�������ӷ���ʽ��ʾCH3COONa��ҺPHΪ8��ԭ�� ��
����Һ�У�C��OH-��= + ��C��Na+��= + ��
��3��ʵ����������FeCl3����Һʱ������FeCl3���������� �У�Ȼ����������ˮϡ�͵������Ũ�ȣ���������ˮ�⣬����õ����ǻ��ǵ���Һ��
��2����֪������0.1mol?L-1 CH3COONa��ҺPHԼΪ8
�������ӷ���ʽ��ʾCH3COONa��ҺPHΪ8��ԭ��
����Һ�У�C��OH-��=
��3��ʵ����������FeCl3����Һʱ������FeCl3����������
���㣺�����ʱ�Ķ����жϼ��й�ph�ļ���,����ˮ���Ӧ��
ר�⣺����ƽ������Һ��pHר��,�����ˮ��ר��
��������1������25��ʱ��ˮ��Һ�д������ӻ�����Kw=10-14���㣬ˮ�������������Ũ�Ⱥ�����������Ũ����ͬ������
��1������25��ʱ��ˮ��Һ�д������ӻ�����Kw=10-14���㣬ˮ�������������Ũ�Ⱥ�����������Ũ����ͬ������
��2����CH3COONa��ҺPH=8����Ϊ��Һ�д��������ˮ����ˮ������������ӣ��ٽ�ˮ����ƽ��������У���Һ������������Ũ������
�ڴ�������Һ�� ���������غ㣬�����غ������
��3���Ȼ�����Һ��������ˮ����Һ�����ԣ������Ȼ�����ҺΪ��ֹˮ������ܽ���Ũ������Һ���ټ�ˮϡ�͵õ�����Ũ�ȵ���Һ��
��1������25��ʱ��ˮ��Һ�д������ӻ�����Kw=10-14���㣬ˮ�������������Ũ�Ⱥ�����������Ũ����ͬ������
��2����CH3COONa��ҺPH=8����Ϊ��Һ�д��������ˮ����ˮ������������ӣ��ٽ�ˮ����ƽ��������У���Һ������������Ũ������
�ڴ�������Һ�� ���������غ㣬�����غ������
��3���Ȼ�����Һ��������ˮ����Һ�����ԣ������Ȼ�����ҺΪ��ֹˮ������ܽ���Ũ������Һ���ټ�ˮϡ�͵õ�����Ũ�ȵ���Һ��
���
�⣺��1��25��ʱ��pH=12.0��KOH��Һ�У���ˮ�������C��OH-��=C��H+��=10-12mol/L��pH=12.0��K2CO3��Һ�У�CO32-+H2O?HCO3-+OH-��Kw=c��H+��c��OH-������ˮ���������C��OH-��=
=
=10-2mol/L����ˮ�������C��OH-��֮��=10-12mol/L��10-2mol/L=1��1010��
�ʴ�Ϊ��1��1010��
��2����CH3COONa��ҺPH=8����Ϊ��Һ�д��������ˮ����ˮ������������ӣ��ٽ�ˮ����ƽ��������У���Һ������������Ũ������ˮ�����ӷ���ʽΪ��CH3COO-+H2O?CH3COOH+OH-��
�ʴ�Ϊ��CH3COO-+H2O?CH3COOH+OH-��
����Һ�д��������ӣ����������ӣ���������ӣ������ӣ�������Һ�������غ�������غ����д���������غ�Ϊ���������Ԫ���غ㣺C��Na+��=C��CH3COOH��+C��CH3COO-���������غ�Ϊˮ����������Ӻ��������غ㣬Ũ�ȹ�ϵΪ��C��OH-��=C��H+��+C��CH3COOH����
�ʴ�Ϊ��C��H+����C��CH3COOH���� C��CH3COOH����C��CH3COO-����
��3���Ȼ�����Һ��������ˮ����Һ�����ԣ�ˮ�����ӷ���ʽΪ��Fe3++3H2O?Fe��OH��3+3H+����ҺPHС��7�������Ȼ�����ҺΪ��ֹˮ������ܽ���Ũ������Һ���ټ�ˮϡ�͵õ�����Ũ�ȵ���Һ��
�ʴ�Ϊ����Ũ�����
| 10-14 |
| c(H+) |
| 10-14 |
| 10-12 |
�ʴ�Ϊ��1��1010��
��2����CH3COONa��ҺPH=8����Ϊ��Һ�д��������ˮ����ˮ������������ӣ��ٽ�ˮ����ƽ��������У���Һ������������Ũ������ˮ�����ӷ���ʽΪ��CH3COO-+H2O?CH3COOH+OH-��
�ʴ�Ϊ��CH3COO-+H2O?CH3COOH+OH-��
����Һ�д��������ӣ����������ӣ���������ӣ������ӣ�������Һ�������غ�������غ����д���������غ�Ϊ���������Ԫ���غ㣺C��Na+��=C��CH3COOH��+C��CH3COO-���������غ�Ϊˮ����������Ӻ��������غ㣬Ũ�ȹ�ϵΪ��C��OH-��=C��H+��+C��CH3COOH����
�ʴ�Ϊ��C��H+����C��CH3COOH���� C��CH3COOH����C��CH3COO-����
��3���Ȼ�����Һ��������ˮ����Һ�����ԣ�ˮ�����ӷ���ʽΪ��Fe3++3H2O?Fe��OH��3+3H+����ҺPHС��7�������Ȼ�����ҺΪ��ֹˮ������ܽ���Ũ������Һ���ټ�ˮϡ�͵õ�����Ũ�ȵ���Һ��
�ʴ�Ϊ����Ũ�����
���������⿼����ˮ�ĵ���ƽ��Ӱ�����ط��������ӻ�����Ӱ�����غͼ��㣬�������Һ������Ũ�ȴ�С�������غ㡢�����غ�ķ���Ӧ�ã����ջ����ǹؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 �¾�����ĩ���100��ϵ�д�
�¾�����ĩ���100��ϵ�д� ȫ�ܴ���100��ϵ�д�
ȫ�ܴ���100��ϵ�д�
�����Ŀ
��NA���������ӵ�����������˵����ȷ���ǣ�������
| A����0.2NA����ԭ�ӵĵ������ʵ���Ϊ0.1mol |
| B����״���£�18gˮ�����е����Ϊ22.4L |
| C��11.2L���������ķ�����ĿΪ0.5NA |
| D��1mol��NaCl�ܽ���1L��ˮ�п��Եõ�1mol/L��NaCl��Һ |
��֪����Ķ��ȴ����ж����칹�壬�������ȴ�����칹����ĿΪ��������
| A������ | B������ | C������ | D������ |