��Ŀ����
�±���Ԫ�����ڱ���һ���֣�������ĸ�ֱ����һ�ֻ�ѧԪ�أ�
�Իش��������⣺
��1����д����Ԫ��o�Ļ�̬ԭ�ӵ����Ų�ʽ ��
��2��k�ڿ�����ȼ�ղ���ķ��ӹ���Ϊ ������ԭ�ӵ��ӻ���ʽΪ ���÷����� ������ԡ��Ǽ��ԡ������ӣ�
��3����10���ӵ�d���⻯����ӵ�VSEPRģ��Ϊ ��
��4��g��h��i����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ ����Ԫ�ط��ţ�
��5������Ԫ�����е縺�������� ����ͼ�е���Ż�Ԫ�ط��ţ���Ԫ��k��������������Ƕ�Ӧ��ˮ���������ǿ��˳��Ϊ �����ѧʽ��
| a | |||||||||||||||||
| b | c | d | e | f | |||||||||||||
| g | h | i | j | k | l | m | |||||||||||
| n | o | ||||||||||||||||
��1����д����Ԫ��o�Ļ�̬ԭ�ӵ����Ų�ʽ
��2��k�ڿ�����ȼ�ղ���ķ��ӹ���Ϊ
��3����10���ӵ�d���⻯����ӵ�VSEPRģ��Ϊ
��4��g��h��i����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ
��5������Ԫ�����е縺��������
���㣺Ԫ�������ɺ�Ԫ�����ڱ����ۺ�Ӧ��
ר�⣺Ԫ����������Ԫ�����ڱ�ר��
��������Ԫ�������ڱ���λ�ã���֪aΪH��bΪLi��cΪC��dΪN��eΪO��fΪF��gΪNa��hΪMg��iΪAl��jΪSi��KΪS��lΪCl��mΪAr��nΪK��oΪFe��
��1��oΪFe��ԭ�Ӻ�����26�����ӣ������������ԭ����д���������Ų�ʽ��
��2��kΪS���ڿ�����ȼ�ղ���ΪSO2������Sԭ�ӹµ��Ӷ������۲���Ӷ�����ȷ�����ӹ��ͣ�Sԭ�ӵ��ӻ���ʽ����Ϸ��ӽṹ�жϷ�����������������Ƿ��غϣ��жϷ��Ӽ��ԣ�
��3����10���ӵ�d���⻯�����ΪNH3������Nԭ�Ӽ۲���Ӷ�����ȷ����VSEPRģ�ͣ�
��4��ͬ�����������Ԫ�ص�һ�����ܳ��������ƣ���MgԪ��ԭ��3s�ܼ�����2�ĵ��ӣ�Ϊȫ���ȶ�״̬�������ϵͣ���һ�����ܸ���Al��
��5������Ԫ�����е縺�������Ƿ���Ԫ��k������������ֱ�Ϊ�������������������Ƕ�Ӧ��ˮ����ֱ�Ϊ�����ᡢ���ᣬ�������������ᣬ����������ǿ�ᣮ
��1��oΪFe��ԭ�Ӻ�����26�����ӣ������������ԭ����д���������Ų�ʽ��
��2��kΪS���ڿ�����ȼ�ղ���ΪSO2������Sԭ�ӹµ��Ӷ������۲���Ӷ�����ȷ�����ӹ��ͣ�Sԭ�ӵ��ӻ���ʽ����Ϸ��ӽṹ�жϷ�����������������Ƿ��غϣ��жϷ��Ӽ��ԣ�
��3����10���ӵ�d���⻯�����ΪNH3������Nԭ�Ӽ۲���Ӷ�����ȷ����VSEPRģ�ͣ�
��4��ͬ�����������Ԫ�ص�һ�����ܳ��������ƣ���MgԪ��ԭ��3s�ܼ�����2�ĵ��ӣ�Ϊȫ���ȶ�״̬�������ϵͣ���һ�����ܸ���Al��
��5������Ԫ�����е縺�������Ƿ���Ԫ��k������������ֱ�Ϊ�������������������Ƕ�Ӧ��ˮ����ֱ�Ϊ�����ᡢ���ᣬ�������������ᣬ����������ǿ�ᣮ
���
�⣺��Ԫ�������ڱ���λ�ã���֪aΪH��bΪLi��cΪC��dΪN��eΪO��fΪF��gΪNa��hΪMg��iΪAl��jΪSi��KΪS��lΪCl��mΪAr��nΪK��oΪFe��
��1��oΪFe��ԭ�Ӻ�����26�����ӣ������������ԭ�������������Ų�ʽΪ1s22s22p63s23p63d64s2��
�ʴ�Ϊ��1s22s22p63s23p63d64s2��
��2��kΪS���ڿ�����ȼ�ղ���ΪSO2��Sԭ�ӹµ��Ӷ���=
=1���۲���Ӷ���=2+1=3���ʷ��ӹ���ΪV�Σ�Sԭ�ӵ��ӻ���ʽΪsp2������������������IJ����غϣ����ڼ��Է��ӣ�
�ʴ�Ϊ��V�Σ�sp2�����ԣ�
��3����10���ӵ�d���⻯�����ΪNH3��Nԭ�Ӽ۲���Ӷ���=3+
=4����VSEPRģ��Ϊ�������壬�ʴ�Ϊ���������壻
��4��ͬ�����������Ԫ�ص�һ�����ܳ��������ƣ���MgԪ��ԭ��3s�ܼ�����2�ĵ��ӣ�Ϊȫ���ȶ�״̬�������ϵͣ���һ�����ܸ���Al���ʵ�һ�����ܣ�M g��Al��Na���ʴ�Ϊ��M g��Al��Na��
��5������Ԫ�����е縺�������Ƿ���Ԫ��k������������ֱ�Ϊ�������������������Ƕ�Ӧ��ˮ����ֱ�Ϊ�����ᡢ���ᣬ�������������ᣬ����������ǿ�ᣬ�����ԣ�H2SO4��H2SO3��
�ʴ�Ϊ��F��H2SO4��H2SO3��
��1��oΪFe��ԭ�Ӻ�����26�����ӣ������������ԭ�������������Ų�ʽΪ1s22s22p63s23p63d64s2��
�ʴ�Ϊ��1s22s22p63s23p63d64s2��
��2��kΪS���ڿ�����ȼ�ղ���ΪSO2��Sԭ�ӹµ��Ӷ���=
| 6-1��2 |
| 2 |
�ʴ�Ϊ��V�Σ�sp2�����ԣ�
��3����10���ӵ�d���⻯�����ΪNH3��Nԭ�Ӽ۲���Ӷ���=3+
| 5-1��3 |
| 2 |
��4��ͬ�����������Ԫ�ص�һ�����ܳ��������ƣ���MgԪ��ԭ��3s�ܼ�����2�ĵ��ӣ�Ϊȫ���ȶ�״̬�������ϵͣ���һ�����ܸ���Al���ʵ�һ�����ܣ�M g��Al��Na���ʴ�Ϊ��M g��Al��Na��
��5������Ԫ�����е縺�������Ƿ���Ԫ��k������������ֱ�Ϊ�������������������Ƕ�Ӧ��ˮ����ֱ�Ϊ�����ᡢ���ᣬ�������������ᣬ����������ǿ�ᣬ�����ԣ�H2SO4��H2SO3��
�ʴ�Ϊ��F��H2SO4��H2SO3��
�����������֪Ԫ�����ڱ���Ԫ�������ɡ����ӽṹ�����ʡ���������Ų��ȣ��Ѷ��еȣ�ע���������շ��ӽṹ���жϣ�

��ϰ��ϵ�д�
 �Ͻ�ƽ���Ȿϵ�д�
�Ͻ�ƽ���Ȿϵ�д� ����ѧ��Ӧ�����ϵ�д�
����ѧ��Ӧ�����ϵ�д�
�����Ŀ
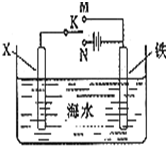 ��1����ʵ֤��������Ƴ�ԭ��صķ�Ӧͨ���Ƿ��ȷ�Ӧ�����л�ѧ��Ӧ�������Ͽ�����Ƴ�ԭ��ص���
��1����ʵ֤��������Ƴ�ԭ��صķ�Ӧͨ���Ƿ��ȷ�Ӧ�����л�ѧ��Ӧ�������Ͽ�����Ƴ�ԭ��ص���