��Ŀ����
18��ij��ѧ��ѧ̽���С�����������ʵ��̽������ijʵ���ҵķ�Һ���ⶨ����Ҫ�����Ҵ�������б�ͪ��������������������ϸ����ʵķе����£�
| ���� | ��ͪ | �������� | �Ҵ� | ���� |
| �е�/�� | 56.2 | 77.06 | 78 | 117.9 |
��l�������ռ�ʹ��Һ�� pH=10��Ŀ����ʹ�����������ˮ�⣬�к�����
��2����70�桫85��ʱ��������Ҫ�ɷ�Ϊ�Ҵ�
��3���ڲ�����м��������Ũ�����Ŀ���ǣ��û�ѧ����ʽ��ʾ��2CH3COONa+H2SO4��Ũ����Na2SO4+2CH3COOH
��4�����������¶ȿ�����85�桫125��֮�䣬����һ��ʱ�������ƿ�ڲ���Һ�����ʵ���Ҫ�ɷ��������ơ����ᣮ
��Ϊ̽������Һ�巢����Ӧ��ԭ��������ͼװ�ý���ʵ�飮���г�װ������ȥ��
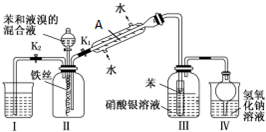
��֪������Һ��ķ�Ӧ�Ƿ��ȷ�Ӧ��
�������֪ʶ�ش��������⣺
��1��ʵ�鿪ʼʱ���ر�K2������K1�ͷ�Һ©���������μӱ���Һ��Ļ��Һ����Ӧ��ʼ������е��л�����������Ӧ�Ļ�ѧ����ʽΪ��

��2������A������Ϊ�������ܣ�����ʵ��װ�����ܷ�ֹ������װ����III��IV����װ����ţ���
��3����Ӧ��������K2���ر�K1�ͷ�Һ©��������ʹװ��I�е�ˮ������װ�â��У�����������Ŀ���Ƿ�Ӧ������װ��II�д��ڴ������廯�⣬ʹI��ˮ������II�п��Գ�ȥ�廯�����壬�����ݳ���Ⱦ������
��4����Ӧ������Ϊ�˵õ��ϴ������屽���Ԣ��е���Һ�����в�������ᴿ��
��������10mLˮ��8mL10%��NaOH��Һ��10mLˮϴ�ӣ�NaOH��Һϴ�ӵ������ǣ���ȥ�屽�����е�HBr��Br2
��ϴ�Ӻ��ٸ�����õ����屽���˴��屽�л����е���Ҫ����Ϊ����Ҫ��һ���ᴿ�����в����б������C��������ȷѡ��ǰ����ĸ����
A���ؽᾧ B������ C������ D����ȡ
��5����װ���Ƿ����жϱ����巢������ȡ����Ӧ���ǣ����еĹ��ƿ�г��ֵ���ɫ����
����ǡ���д����˵����������ȡ����Ӧ����������������ԭ��
���� �����ռ�ʹ��Һ��pH=10�����Ҵ��е�������������������ת���������ƶ����������Ҵ�����Һ����Ҫ����������Һ����ȴ�������м�Ũ���ᣨ����������������ת�������ᣬ�������ռ����ᣬ�Դ˽�ɣ�
��1�������ռ�ʹ��Һ��pH=10�����Ҵ��е�������������������ת���������ƶ�������룻
��2���������Ϸ������Ҵ��ķе�78�������
��3���������Ϸ������ڲ�����У��������Ũ�����Ŀ���ǽ�������ת�������
��4���������Ϸ���������ķе�117.9�������
��1���ر�K2������K1�ͷ�Һ©���������μӱ���Һ��Ļ��Һ��Һ������˿��Ӧ����FeBr3��FeBr3������Һ�巢����Ӧ�����屽���廯�⣻��2������A������Ϊ�����ܣ�װ��III�Թ��ﱽ�뵼�ܵ��ܿڽӴ��������Сװ���ڵ�����ѹǿ��IV�ձ��У������ĸ�����ݻ�����������ˮ������������У�ʹ�ձ���Һ���½���������Һ���������������»������ձ��У�
��3��װ��II�к����廯����������Ⱦ������
��4���٢��е��屽��������������廯�⣬������NaOH��Һ��ȥ��
�ڱ��������屽�У��Һ�����������Һ����Ӧ�����Դ��屽�л����е���Ҫ����Ϊ������ȥ������������ķ�����
��5���������ȡ����Ӧ������廯�⣬��������ӳɷ�Ӧ��������廯�⣬��ֻҪ�������������Ӳ������ɣ��ݴ˷�����
��� �⣺�����ռ�ʹ��Һ��pH=10�����Ҵ��е�������������������ת���������ƶ����������Ҵ�����Һ����Ҫ����������Һ����ȴ�������м�Ũ���ᣨ����������������ת�������ᣬ�������ռ����
��1�������ռ�ʹ��Һ��pH=10�����Ҵ��е�������������������ת���������ƶ�������룬�����ᷴӦ���������ƣ�������������Ӧ�����Ҵ��������ƣ���Ӧ����ʽΪ��CH3COOH+NaOH��CH3COONa+H2O��CH3COOCH2CH3+NaOH��CH3COONa+CH3CH2OH�����Լ����ռ�ʹ��Һ�� pH=10��Ŀ����ʹ�����������ˮ�⣬�к�����ʴ�Ϊ��ʹ�����������ˮ�⣬�к����
��2���������Ϸ������Ҵ��ķе�78�棬������70��85��ʱ��������Ҫ�ɷ����Ҵ����ʴ�Ϊ���Ҵ���
��3�������Ϸ������ڲ�����У��������Ũ�����Ŀ���ǽ�������ת�������ᣬ����ʽΪ��2CH3COONa+H2SO4��Ũ����Na2SO4+2CH3COOH���ʴ�Ϊ��2CH3COONa+H2SO4��Ũ����Na2SO4+2CH3COOH��
��4���������Ϸ���������ķе�117.9�棬���Բ���Һ�����ʵ���Ҫ�ɷ��������ơ����ᣬ�ʴ�Ϊ�������ơ����
��1���ر�K2������K1�ͷ�Һ©���������μӱ���Һ��Ļ��Һ��Һ������˿��Ӧ����FeBr3��FeBr3������Һ�巢����Ӧ�����屽���廯�⣬����е��л�����������Ӧ�Ļ�ѧ����ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2������A������Ϊ�����ܣ�װ��III�Թ��ﱽ�뵼�ܿڽӴ�������Ӱ��װ���ڵ�����ѹǿ�����Կ��Է�ֹ����������IV�ձ��У������ĸ�����ݻ�����������ˮ������������У�ʹ�ձ���Һ���½���������Һ���������������»������ձ��У����Կ��Է�ֹ����������
�ʴ�Ϊ�������ܣ�III��IV��
��3����װ��II�к����廯����������Ⱦ��������Ӧ������װ��II�д��ڴ������廯�⣬ʹI��ˮ������II�п��Գ�ȥ�廯�����壬�����ݳ���Ⱦ�������ʴ�Ϊ����Ӧ������װ��II�д��ڴ������廯�⣬ʹI��ˮ������II�п��Գ�ȥ�廯�����壬�����ݳ���Ⱦ������
��4���������屽�������廯���뵥���壬��������������Һ������������HBr��Br2��������Ӧ�Ļ�ѧ����ʽΪBr2+2NaOH=NaBr+NaBrO+H2O��HBr+NaOH=NaBr+H2O������NaOH��Һϴ�ӵ������dz�ȥ�屽�����е�HBr��Br2���ʴ�Ϊ����ȥ�屽�����е�HBr��Br2��
�ڱ��������屽�У��Һ�����������Һ����Ӧ�����Դ��屽�л����е���Ҫ����Ϊ�������ߵķе����ϴ����ɷ��룬��ѡC���ʴ�Ϊ������C
��5��ȡ����Ӧ�ͼӳɷ�Ӧ�����������Ƿ�����廯�⣬ͨ�����еĹ��ƿ�г��ֵ���ɫ��������֤�������������ӣ����������ܷ���ȡ����Ӧ����HBr���ʴ�Ϊ���ǣ����еĹ��ƿ�г��ֵ���ɫ������
���� ����ͨ��ʵ����Ҫ�����˻����ķ�����ᴿ�Լ��������ʣ���Ŀ�Ѷ��еȣ�ע��ȷʵ��Ŀ�ĺ�ʵ��ԭ���Լ���Ϥ�����������ص����;�ǽ���Ĺؼ��������ֿ�����ѧ���ķ�����������������ѧʵ��������

| A�� | 1.8��10-7 mol•L-1 | B�� | 1.0��10-5 mol•L-1 | ||
| C�� | 1.0��10-7 mol•L-1 | D�� | 1.8��10-9 mol•L-1 |

| A�� | Ԫ�ص�һ�����ܴ�С��J��R��Q | |
| B�� | ��TԪ�ص������ε���Һ��һ���������Ӧ | |
| C�� | Z�ĺ�����һ��Ϊǿ�� | |
| D�� | Q��R��J��Ԫ����J�ĵ縺����� |
| A�� | �ܱ������ж�����̼������������� | |
| B�� | �ܱ�����������������ʵ����ı� | |
| C�� | �ܱ������л��������ܶȲ��� | |
| D�� | 2v��NH3����=v��CO2���� |
| A�� | ������Һ��Na+��NH4+��SO42-��NO3- | B�� | FeCl3��Һ��K+��Na+��SCN-��CO32- | ||
| C�� | ������Һ��K+��Na+��SO42-��S2O32- | D�� | ��ɫ��Һ��K+��Na+��NO3-��SO42- |
| A�� | 2 g ���������е�ԭ����Ŀ��0.25 NA | |
| B�� | ���³�ѹ�£�0.5 mol Cl2��ռ�е����ԼΪ11.2 L | |
| C�� | ��״���£�0.9 mLˮ������ԼΪ0.9 g | |
| D�� | 0.5 mol/L Na2SO4 ��Һ��Na+ �����ʵ�����1 mol |
| A�� | ���������ԣ�����Ϊ�ǻ��Ա���Ӱ��Ľ�� | |
| B�� | ����ʽΪC2H4��C3H6������һ����Ϊͬϵ�� | |
| C�� | �ۺ���  �DZ��Ӻͼ�ȩ��һ�������·�Ӧ�õ��ĸ߷��ӻ����� �DZ��Ӻͼ�ȩ��һ�������·�Ӧ�õ��ĸ߷��ӻ����� | |
| D�� | 1mol ��������NaOH��Һ���ȳ�ַ�Ӧ�����������4mol NaOH ��������NaOH��Һ���ȳ�ַ�Ӧ�����������4mol NaOH |
| A�� | CuS | B�� | FeS | C�� | ZnS | D�� | MgS |