��Ŀ����
����73gij���壬��������ԭ�ӹ��ɵķ��ӣ�����Ħ������Ϊ36.5g?mol-1���������ӵ�������NA��ʾ����
��1������������ʵ���Ϊ mol��
��2������������ԭ������Ϊ ����
��3���������ڱ�״���µ����Ϊ L��
��4������������1Lˮ�У�����Һ�����ʵ���������Ϊ ��
��5��1���ˮ�ܽ������500�������������Һ�������ˮ���������������Һ�����ʵ���Ũ��Ϊ mol?L-1��
��1������������ʵ���Ϊ
��2������������ԭ������Ϊ
��3���������ڱ�״���µ����Ϊ
��4������������1Lˮ�У�����Һ�����ʵ���������Ϊ
��5��1���ˮ�ܽ������500�������������Һ�������ˮ���������������Һ�����ʵ���Ũ��Ϊ
���㣺���ʵ�������ؼ���
ר�⣺������
��������1������n=
������������ʵ�����
��2��ԭ�����ʵ���Ϊ�����2�����ٸ���N=nNA���㣻
��3������V=nVm��������������
��4������m=��V����ˮ������������������Һ�������ٸ�����������������㣻
��5����ˮΪ1L��������Ϊ500L����Һ���Ϊ1L������n=
������������ʵ������ٸ���c=
����������Һ���ʵ���Ũ�ȣ�
| m |
| M |
��2��ԭ�����ʵ���Ϊ�����2�����ٸ���N=nNA���㣻
��3������V=nVm��������������
��4������m=��V����ˮ������������������Һ�������ٸ�����������������㣻
��5����ˮΪ1L��������Ϊ500L����Һ���Ϊ1L������n=
| V |
| Vm |
| n |
| V |
���
�⣺��1�������Ħ������Ϊ36.5g?mol-1��73g��������ʵ���=
=2mol���ʴ�Ϊ��2��
��2��ԭ�����ʵ���Ϊ2mol��2=4mol���ʺ���ԭ����ĿΪ4NA���ʴ�Ϊ��4NA��
��3������£�����������=2mol��22.4L/mol=44.8L���ʴ�Ϊ��44.8��
��4��1Lˮ������Ϊ1000mL��1g/mL=1000g����Һ��������=
��100%=6.8%���ʴ�Ϊ��6.8%��
��5����ˮΪ1L��������Ϊ500L����Һ���Ϊ1L����������ʵ���=
=
mol��������Һ���ʵ���Ũ��=
=22.3mol/L���ʴ�Ϊ��22.3��
| 73g |
| 36.5g/mol |
��2��ԭ�����ʵ���Ϊ2mol��2=4mol���ʺ���ԭ����ĿΪ4NA���ʴ�Ϊ��4NA��
��3������£�����������=2mol��22.4L/mol=44.8L���ʴ�Ϊ��44.8��
��4��1Lˮ������Ϊ1000mL��1g/mL=1000g����Һ��������=
| 73g |
| 1000g+73g |
��5����ˮΪ1L��������Ϊ500L����Һ���Ϊ1L����������ʵ���=
| 500L |
| 22.4L/mol |
| 500 |
| 22.4 |
| ||
| 1L |
���������⿼�����ʵ����йؼ��㣬�ѶȲ���ע�����������ʵ���Ϊ���ĵļ��㹫ʽ��

��ϰ��ϵ�д�
 ȫ�ܲ��һ���þ�ϵ�д�
ȫ�ܲ��һ���þ�ϵ�д�
�����Ŀ
��4mol A��g����2mol B��g����2L�����ڻ�ϣ���һ�������·�Ӧ��2A��g��+B��g��?2C��g������2s����C��Ũ��Ϊ0.6mol/L�������м���˵����������ȷ���ǣ�������
��1��������A��ʾ�ķ�Ӧ��ƽ������Ϊ0.3mol/��L?s��
��2��������B��ʾ�ķ�Ӧ��ƽ������Ϊ0.6mol/��L?s��
��3��2sʱ����Aת����Ϊ15%
��4��2sʱ����B��Ũ��Ϊ0.7mol/L��
��1��������A��ʾ�ķ�Ӧ��ƽ������Ϊ0.3mol/��L?s��
��2��������B��ʾ�ķ�Ӧ��ƽ������Ϊ0.6mol/��L?s��
��3��2sʱ����Aת����Ϊ15%
��4��2sʱ����B��Ũ��Ϊ0.7mol/L��
| A����1����3�� |
| B����1����4�� |
| C����1����3����4�� |
| D����2����3����4�� |
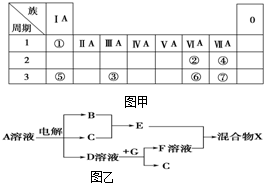 ��ͼ��ΪԪ�����ڱ���һ���֣�����Ԫ�آ١����ڱ��е�λ�ã����û�ѧ����ش��������⣺
��ͼ��ΪԪ�����ڱ���һ���֣�����Ԫ�آ١����ڱ��е�λ�ã����û�ѧ����ش��������⣺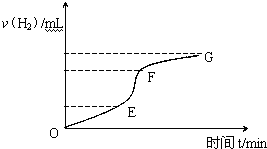 �ô�����п����ϡ���ᷴӦ��ȡ�������壬��ش�
�ô�����п����ϡ���ᷴӦ��ȡ�������壬��ش� ��һ���¶��£��������ˮϡ�����У���Һ�ĵ���������ͼ��
��һ���¶��£��������ˮϡ�����У���Һ�ĵ���������ͼ��