��Ŀ����
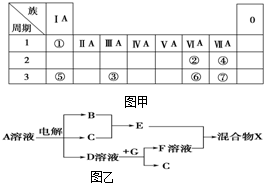 ��ͼ��ΪԪ�����ڱ���һ���֣�����Ԫ�آ١����ڱ��е�λ�ã����û�ѧ����ش��������⣺
��ͼ��ΪԪ�����ڱ���һ���֣�����Ԫ�آ١����ڱ��е�λ�ã����û�ѧ����ش��������⣺��1���ܡ��ݡ��ߵ�ԭ�Ӱ뾶�ɴ�С��˳��Ϊ
��2���͢ߵ���ۺ����������ǿ��Ϊ
��3���ܡ��ޡ��ߵ���̬�⻯����ȶ���������ǿ��˳����
��4���١�������Ԫ�ذ�ԭ�Ӹ���֮��Ϊ1��1��ɵij���Һ̬�������������Һ���ܽ�Fe2+������д���÷�Ӧ�����ӷ���ʽ
��5���ɱ���Ԫ���γɵ����ʿɷ�����ͼ���еķ�Ӧ������B��C��G�ǵ��ʣ�BΪ����ɫ���壬D��Һ�Լ��ԣ�
��д��D��Һ��G��Ӧ�Ļ�ѧ����ʽ
��д������A��Һ�����ʵ������ӵķ�����
����ͼ���и�����Ӧ��Ϊǡ����ȫת����������X�к��е�������
���㣺Ԫ�������ɺ�Ԫ�����ڱ����ۺ�Ӧ��
ר�⣺Ԫ����������Ԫ�����ڱ�ר��
��������Ԫ�������ڱ���λ�ã���֪��ΪH����ΪO����ΪAl����ΪF����ΪNa����ΪS����ΪCl��
��1��ͬ�����������ԭ�Ӱ뾶��С��ͬ�������϶���ԭ�Ӱ뾶����
��2���ǽ�����Խǿ����ۺ����������Խǿ��
��3���ǽ�����Խǿ���⻯��Խ�ȶ���
��4���١�������Ԫ�ذ�ԭ�Ӹ���֮��Ϊ1��1��ɵij���Һ̬������ΪH2O2+����������Һ���ܽ�Fe2+��������Fe3+����������ԭΪH2O��
��5��B��C��G�ǵ��ʣ�BΪ����ɫ���壬��B��Cl2��D��Һ�Լ��ԣ�Ӧ�ǵ�ⱥ���Ȼ�����Һ����A��NaCl��C��H2��D��NaOH��E��HCl���������ƺ͵���G��Ӧ����������F����G��Al��F��NaAlO2����ⷴӦΪ2NaCl+2H2O
2NaOH+H2��+Cl2������2Al��2NaOH��2NaAlO2���õ�ƫ��������HCl���ʵ���֮��Ϊ1��1����H2O+HCl+NaAlO2�TNaCl+Al ��OH��3������X�к��е�����ΪAl ��OH��3��H2O��NaCl��
��1��ͬ�����������ԭ�Ӱ뾶��С��ͬ�������϶���ԭ�Ӱ뾶����
��2���ǽ�����Խǿ����ۺ����������Խǿ��
��3���ǽ�����Խǿ���⻯��Խ�ȶ���
��4���١�������Ԫ�ذ�ԭ�Ӹ���֮��Ϊ1��1��ɵij���Һ̬������ΪH2O2+����������Һ���ܽ�Fe2+��������Fe3+����������ԭΪH2O��
��5��B��C��G�ǵ��ʣ�BΪ����ɫ���壬��B��Cl2��D��Һ�Լ��ԣ�Ӧ�ǵ�ⱥ���Ȼ�����Һ����A��NaCl��C��H2��D��NaOH��E��HCl���������ƺ͵���G��Ӧ����������F����G��Al��F��NaAlO2����ⷴӦΪ2NaCl+2H2O
| ||
���
�⣺��Ԫ�������ڱ���λ�ã���֪��ΪH����ΪO����ΪAl����ΪF����ΪNa����ΪS����ΪCl��
��1��ͬ�����������ԭ�Ӱ뾶��С��ͬ�������϶���ԭ�Ӱ뾶����ԭ�Ӱ뾶��Na��Cl��F���ʴ�Ϊ��Na��Cl��F��
��2���ǽ�����Cl��S�����Ӧ����������������ΪHClO4��H2SO4���ʴ�Ϊ��HClO4��H2SO4��
��3���ǽ�����F��Cl��S���ǽ�����Խǿ���⻯��Խ�ȶ������⻯���ȶ��ԣ�H2S��HCl��HF���ʴ�Ϊ��H2S��HCl��HF��
��4���١�������Ԫ�ذ�ԭ�Ӹ���֮��Ϊ1��1��ɵij���Һ̬������ΪH2O2+����������Һ���ܽ�Fe2+��������Fe3+����������ԭΪH2O����Ӧ���ӷ���ʽΪ��H2O2+2Fe2++2H+�T2Fe3++2H2O���ʴ�Ϊ��H2O2+2Fe2++2H+�T2Fe3++2H2O��
��5��B��C��G�ǵ��ʣ�BΪ����ɫ���壬��B��Cl2��D��Һ�Լ��ԣ�Ӧ�ǵ�ⱥ���Ȼ�����Һ����A��NaCl��C��H2��D��NaOH��E��HCl���������ƺ͵���G��Ӧ����������F����G��Al��F��NaAlO2����H2O+HCl+NaAlO2�TNaCl+Al ��OH��3������X�к��е�����ΪAl ��OH��3��H2O��NaCl��
��D��Һ��G��Ӧ�Ļ�ѧ����ʽΪ��2Al+2NaOH+6H2O�T2Na[Al��OH��4]+3H2�����ʴ�Ϊ��2Al+2NaOH+6H2O�T2Na[Al��OH��4]+3H2����
�ڼ���NaCl��Һ�����ʵ������ӵķ�����ȡ����A��Һ���Թ��У��μӼ��Σ�ϡ�����ữ�ģ���������Һ�а�ɫ�������ɣ��ʴ�Ϊ��ȡ����A��Һ���Թ��У��μӼ��Σ�ϡ�����ữ�ģ���������Һ�а�ɫ�������ɣ�
�۵�ⷴӦΪ2NaCl+2H2O
2NaOH+H2��+Cl2������2Al��2NaOH��2NaAlO2���õ�ƫ��������HCl���ʵ���֮��Ϊ1��1����H2O+HCl+NaAlO2�TNaCl+Al ��OH��3������X�к��е�����ΪAl ��OH��3��H2O��NaCl���ʴ�Ϊ��Al��OH��3��H2O��NaCl��
��1��ͬ�����������ԭ�Ӱ뾶��С��ͬ�������϶���ԭ�Ӱ뾶����ԭ�Ӱ뾶��Na��Cl��F���ʴ�Ϊ��Na��Cl��F��
��2���ǽ�����Cl��S�����Ӧ����������������ΪHClO4��H2SO4���ʴ�Ϊ��HClO4��H2SO4��
��3���ǽ�����F��Cl��S���ǽ�����Խǿ���⻯��Խ�ȶ������⻯���ȶ��ԣ�H2S��HCl��HF���ʴ�Ϊ��H2S��HCl��HF��
��4���١�������Ԫ�ذ�ԭ�Ӹ���֮��Ϊ1��1��ɵij���Һ̬������ΪH2O2+����������Һ���ܽ�Fe2+��������Fe3+����������ԭΪH2O����Ӧ���ӷ���ʽΪ��H2O2+2Fe2++2H+�T2Fe3++2H2O���ʴ�Ϊ��H2O2+2Fe2++2H+�T2Fe3++2H2O��
��5��B��C��G�ǵ��ʣ�BΪ����ɫ���壬��B��Cl2��D��Һ�Լ��ԣ�Ӧ�ǵ�ⱥ���Ȼ�����Һ����A��NaCl��C��H2��D��NaOH��E��HCl���������ƺ͵���G��Ӧ����������F����G��Al��F��NaAlO2����H2O+HCl+NaAlO2�TNaCl+Al ��OH��3������X�к��е�����ΪAl ��OH��3��H2O��NaCl��
��D��Һ��G��Ӧ�Ļ�ѧ����ʽΪ��2Al+2NaOH+6H2O�T2Na[Al��OH��4]+3H2�����ʴ�Ϊ��2Al+2NaOH+6H2O�T2Na[Al��OH��4]+3H2����
�ڼ���NaCl��Һ�����ʵ������ӵķ�����ȡ����A��Һ���Թ��У��μӼ��Σ�ϡ�����ữ�ģ���������Һ�а�ɫ�������ɣ��ʴ�Ϊ��ȡ����A��Һ���Թ��У��μӼ��Σ�ϡ�����ữ�ģ���������Һ�а�ɫ�������ɣ�
�۵�ⷴӦΪ2NaCl+2H2O
| ||
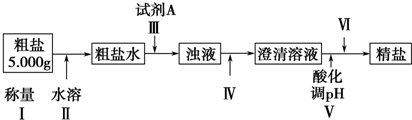
���������⿼��Ԫ�����ڱ���Ԫ�������ɡ�������ƶϣ��Ѷ��еȣ���Ҫѧ���߱���ʵ�Ļ�����

��ϰ��ϵ�д�
 �óɼ�1��1��ĩ���100��ϵ�д�
�óɼ�1��1��ĩ���100��ϵ�д� ��״Ԫ���źþ�ϵ�д�
��״Ԫ���źþ�ϵ�д�
�����Ŀ
��a g�����������Ļ�������800mL pH=1�������г�ַ�Ӧ������ʣ�࣬����ȫ�����ģ��ų���״�������� 0.224L���������ж�����ȷ���ǣ�������
| A��ԭ�������n��Fe����n��Fe2O3��=2��1 |
| B������Һ�е�����ɫ��KSCN��Һ����Ѫ��ɫ |
| C���������ԭ���������� |
| D����ʱ��Һ��Fe2+��Fe3+�����ʵ���֮��Ϊ3��1 |
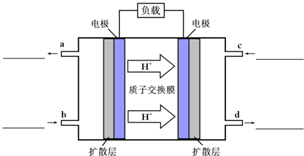 �״�-����ȼ�ϵ�أ�DMFC����һ�ָ�Ч�ܡ�����Ⱦ�綯�����ij��ص�أ���ȼ�ϵ�صĵ�ط�ӦʽΪ��CH3OH��l��+
�״�-����ȼ�ϵ�أ�DMFC����һ�ָ�Ч�ܡ�����Ⱦ�綯�����ij��ص�أ���ȼ�ϵ�صĵ�ط�ӦʽΪ��CH3OH��l��+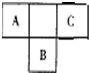 ������Ԫ�ص�A��B��C��Ԫ�����ڱ��е�λ������ͼ��ʾ����֪A��C ����Ԫ�ص�ԭ�Ӻ��������֮�͵���B����������Bԭ�Ӻ�������������������ȣ��ݴ���գ�
������Ԫ�ص�A��B��C��Ԫ�����ڱ��е�λ������ͼ��ʾ����֪A��C ����Ԫ�ص�ԭ�Ӻ��������֮�͵���B����������Bԭ�Ӻ�������������������ȣ��ݴ���գ�