��Ŀ����
 ��һ���¶��£��������ˮϡ�����У���Һ�ĵ���������ͼ��
��һ���¶��£��������ˮϡ�����У���Һ�ĵ���������ͼ����1����O���㵼������Ϊ�������
��2��A��B��C���������ɴ�С��˳����
��3����ʹC����Һ�е�c��CH3COO-����ߣ������´�ʩ�У���ѡ
A������ B���Ӻ�ϡ��NaOH��Һ
C���ӹ���KOH D����ˮ
E���ӹ���CH3COONa F����Zn��
��4����ϡ�����У����Ŵ���Ũ�ȵĽ��ͣ�����ʼ�ձ����������Ƶ�����
A��c��H+�� B��H+���� C��CH3COOH������ D��c��H+��/c��CH3COOH��
���㣺���������ˮ��Һ�еĵ���ƽ��
ר�⣺����ƽ������Һ��pHר��
��������1����Һ�ĵ���ԭ���Ǵ��������ƶ������ӣ�
��2����������Խǿ������Ũ��Խ��������Ũ��Խ��pHԽС��
��3��Ҫʹ���������Ũ�������Բ��ü��ȡ����뺬�д�������ӵ����ʡ�����������ӷ�Ӧ�����ʣ�
��4����ˮϡ�ͣ��ٽ����룬n��CH3COO-����n��H+������Ũ�ȼ�С���Դ˽��
��2����������Խǿ������Ũ��Խ��������Ũ��Խ��pHԽС��
��3��Ҫʹ���������Ũ�������Բ��ü��ȡ����뺬�д�������ӵ����ʡ�����������ӷ�Ӧ�����ʣ�
��4����ˮϡ�ͣ��ٽ����룬n��CH3COO-����n��H+������Ũ�ȼ�С���Դ˽��
���
�⣺��1����Һ�ĵ�����������Ũ���йأ�����Ũ��Խ������Խǿ����������û�������ƶ������ӣ����Ա�������磬
�ʴ�Ϊ����Ϊ������δ���룬�������ƶ������ӣ�
��2����������Խǿ������Ũ��Խ��������Ũ��Խ����a��b��c������Һ��������Ũ����С�����˳��ΪΪc��a��b���ʴ�Ϊ��b��a��c��
��3��A�����ȴٽ�������룬����Һ�д��������Ũ��������ȷ��
B���Ӻ�ϡ��NaOH��Һ���ٽ�������룬����Һ����������´��������Ũ�ȼ�С���ʴ���
C����KOH���壬�������غ������ӷ�Ӧ�ٽ�������룬���Դ��������Ũ��������ȷ��
D����ˮϡ���ܴٽ�������룬�����������Ũ�ȼ�С���ʴ���
E���ӹ���CH3COONa�������ƴ�����룬�������Ƶ�����Ĵ�������Ӵ������ƴ��������Ĵ�������ӣ����Դ��������Ũ��������ȷ��
F������п�����������ӷ�Ӧ���ٽ�������룬���Դ��������Ũ��������ȷ��
�ʴ�Ϊ��A��C��E��F��
��4��A����ϡ�����У���Һ��������������ӵ�Ũ�ȼ�С����A����
B���������Խϡ�������Խ�����������������Խ�࣬��B��ȷ��
C���������Խϡ�������Խ��ƽ�������ƶ���CH3COOH���������٣���C����
D����ˮϡ�ͣ��ٽ����룬n��CH3COO-����n��H+������n��CH3COOH����С�������ֵ������D��ȷ��
�ʴ�Ϊ��B��D��
�ʴ�Ϊ����Ϊ������δ���룬�������ƶ������ӣ�
��2����������Խǿ������Ũ��Խ��������Ũ��Խ����a��b��c������Һ��������Ũ����С�����˳��ΪΪc��a��b���ʴ�Ϊ��b��a��c��
��3��A�����ȴٽ�������룬����Һ�д��������Ũ��������ȷ��
B���Ӻ�ϡ��NaOH��Һ���ٽ�������룬����Һ����������´��������Ũ�ȼ�С���ʴ���
C����KOH���壬�������غ������ӷ�Ӧ�ٽ�������룬���Դ��������Ũ��������ȷ��
D����ˮϡ���ܴٽ�������룬�����������Ũ�ȼ�С���ʴ���
E���ӹ���CH3COONa�������ƴ�����룬�������Ƶ�����Ĵ�������Ӵ������ƴ��������Ĵ�������ӣ����Դ��������Ũ��������ȷ��
F������п�����������ӷ�Ӧ���ٽ�������룬���Դ��������Ũ��������ȷ��
�ʴ�Ϊ��A��C��E��F��
��4��A����ϡ�����У���Һ��������������ӵ�Ũ�ȼ�С����A����
B���������Խϡ�������Խ�����������������Խ�࣬��B��ȷ��
C���������Խϡ�������Խ��ƽ�������ƶ���CH3COOH���������٣���C����
D����ˮϡ�ͣ��ٽ����룬n��CH3COO-����n��H+������n��CH3COOH����С�������ֵ������D��ȷ��
�ʴ�Ϊ��B��D��
�����������ۺϿ������ʵĵ��룬������ѧ���ķ��������Ŀ��飬Ϊ�߿��������ͺ�Ƶ���㣬ע���ˮϡ�ʹ��ᣬ�ܴٽ�������룬����Һ�д���������������ԶԶС��ˮ���������������Դ��������Ũ�ȼ�С��Ϊ�״��㣮

��ϰ��ϵ�д�
 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д�
�����Ŀ
��a g�����������Ļ�������800mL pH=1�������г�ַ�Ӧ������ʣ�࣬����ȫ�����ģ��ų���״�������� 0.224L���������ж�����ȷ���ǣ�������
| A��ԭ�������n��Fe����n��Fe2O3��=2��1 |
| B������Һ�е�����ɫ��KSCN��Һ����Ѫ��ɫ |
| C���������ԭ���������� |
| D����ʱ��Һ��Fe2+��Fe3+�����ʵ���֮��Ϊ3��1 |
���ۼ������Ӽ��������������Ӽ�������������������������������������������ľ����ǣ�������
| A��HCl | B�����ʯ |
| C��NaOH | D��Na |
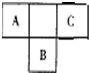 ������Ԫ�ص�A��B��C��Ԫ�����ڱ��е�λ������ͼ��ʾ����֪A��C ����Ԫ�ص�ԭ�Ӻ��������֮�͵���B����������Bԭ�Ӻ�������������������ȣ��ݴ���գ�
������Ԫ�ص�A��B��C��Ԫ�����ڱ��е�λ������ͼ��ʾ����֪A��C ����Ԫ�ص�ԭ�Ӻ��������֮�͵���B����������Bԭ�Ӻ�������������������ȣ��ݴ���գ�
 ��֪��Һ�У���ԭ��HSO3-��I-��������IO3-��I2��SO42-���ں�3molNaHSO3����Һ����μ���KIO3��Һ�������KIO3��������I2�����ʵ����Ĺ�ϵ��������ͼ��ʾ���Իش��������⣺
��֪��Һ�У���ԭ��HSO3-��I-��������IO3-��I2��SO42-���ں�3molNaHSO3����Һ����μ���KIO3��Һ�������KIO3��������I2�����ʵ����Ĺ�ϵ��������ͼ��ʾ���Իش��������⣺