��Ŀ����
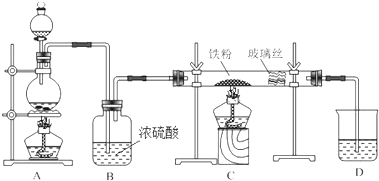
13��ij��������ȡ�����������ͼ��ʾ��
��1����������ͨNH3��ͨCO2��ԭ���ǰ�����ˮ���ܽ�ȴ���ͨ�������������ո���Ķ�����̼���������HCO3-��Ũ�ȣ������ڴٽ������NaHCO3���������ɳ���A�Ļ�ѧ����ʽΪNH3+CO2+H2O+NaCl=NH4Cl+NaHCO3����
��2�������п�ѭ�����õ�����ΪCO2��NaCl�����������Լ�����������и���ƷF�Ƿ��IJ�����ȡ�����Ȼ�鱗�Ʒ���Թܵײ������ȣ����Թܵײ�����������Ȼ�鱗�Ʒ������
��3���ϳɰ�������900��1000��ʱ��ʹ����Ȼ����ˮ�����ڴ�����Al2O3+Ni�������»�úϳɰ���ԭ����֮һ�������÷�Ӧ�Ļ�ѧ����ʽΪCH4+H2O$\frac{\underline{\;\;\;\;\;Al_{2}O_{3}+Ni\;\;\;\;\;}}{900��1000��}$CO2+4H2��CH4+H2O$\frac{\underline{\;\;\;\;\;Al_{2}O_{3}+Ni\;\;\;\;\;}}{900��1000��}$CO+3H2��
��4���ϳɰ���ҵʵ��ѡ���������B������ĸ���ţ���
A.200�棬10MPa B.400�棬20MPa C.800�棬5MPa
��5���ڸ������У�ÿʹ��11.70tʳ�����յõ�10.07t�����NaCl��������Ϊ95%��
���� ������Ϊ�����Ƽ�����Ȼ��ƣ�������������̼��ˮΪԭ�ϣ���ʳ��ˮ��ͨ���������������ͣ�Ȼ���ڼ�ѹ��ͨ��CO2����CaCO3���ն��ã�����NaHCO3�ܽ�Ƚ�С���ᾧ�����������ķ�ӦΪ��
NH3+CO2+H2O�TNH4HCO3��NaCl+NH4HCO3�TNaHCO3��+NH4Cl
��������NaHCO3�������գ�����Na2CO3��2NaHCO3=Na2CO3+CO2��+H2O
����NH4Cl �ڳ���ʱ���ܽ�ȱ�NaCl���ڵ�����ȴ��NaCl�ܽ��С��ԭ������278K��283K��5�桫10�棩ʱ����ĸҺ�м���ʳ��ϸ�ۣ���ʹNH4Cl �����ᾧ�����������ʣ�
��1��������ˮ��Һ���ܽ�Ⱥܴ��Ȼ�����Һ���հ��������ܽ����Ķ�����̼�����ݷ�Ӧԭ��д���̣�
��2�����ԭ����ͼʾ����ѭ��������ΪCO2��NaCl��FΪ�Ȼ�泥������������ֽ��������֤��
��3������������ԭ��Ӧ�Ĺ��ɣ����ϼ������۾��н��ۣ��Ʋ����÷���ʽ��
��4������Ӱ�컯ѧ��Ӧ���ʵ����أ��¶ȡ�Ũ�ȡ�ѹǿ���������Ӵ������Ӱ�컯ѧƽ���ƶ������أ��¶ȡ�Ũ�ȡ�ѹǿ��֪ʶ���ʵ�ʹ�ҵ�������ش�
��5�����ݷ�Ӧ����ʽ�����Ȼ����봿���������������Ȼ��Ƴ������ʱ���ɵĴ�����������ٸ���ʵ�����ɴ�������Ȼ��Ƶ������ʣ�
��� �⣺��1��NH3��NaCl��Һ���ܽ�Ⱥܴ�CO2�ڰ�������Һ���ܽ�ȴ���ͨNH3��ͨCO2���γɸ�Ũ�ȵ�HCO3-������ͨCO2��ͨNH3������CO2��NaCl��Һ���ܽ�Ⱥ�С������NH3��̼�ữ����Һ���ܽ�ȴ�Ҳ�����γɸ�Ũ�ȵ�HCO3-�����ݷ�Ӧԭ����NH3+CO2+H2O�TNH4HCO3��NaCl+NH4HCO3�TNaHCO3��+NH4Cl���������ɳ����ķ���ΪNH3+CO2+H2O+NaCl=NH4Cl+NaHCO3����
�ʴ�Ϊ��������ˮ���ܽ�ȴ���ͨ�������������ո���Ķ�����̼���������HCO3-��Ũ�ȣ������ڴٽ������NaHCO3������NH3+CO2+H2O+NaCl=NH4Cl+NaHCO3����
��2����Ӧ��Ҫ������̼���Ȼ��ƣ����ɵ�̼���������ȷֽ��̼���ơ�������̼��C����������̼��ѭ��ʹ�ã���ĸҺB����ʳ��ϸ�ۣ���ʹNH4Cl �ᾧ������������Һ�Ȼ��ƣ���ѭ��ʹ�ã�FΪNH4Cl���Ȼ�������ֽ⣬���ֽ���ȫ������ƷȫΪ�Ȼ�泥�
�ʴ�Ϊ��CO2��NaCl��ȡ�����Ȼ�鱗�Ʒ���Թܵײ������ȣ����Թܵײ�����������Ȼ�鱗�Ʒ������
��3���ϳɰ�������900��1000��ʱ��ʹ����Ȼ����ˮ�����ڴ�����Al2O3+Ni�������»�úϳɰ���ԭ����֮һ����������������ԭ��Ӧ���ɣ���Ԫ�ػ��ϼ۽��ͣ���̼Ԫ�ػ��ϼ����ߣ���ΪCO2����CO��
�ʴ�Ϊ��CH4+H2O$\frac{\underline{\;\;\;\;\;Al_{2}O_{3}+Ni\;\;\;\;\;}}{900��1000��}$CO2+4H2��CH4+H2O$\frac{\underline{\;\;\;\;\;Al_{2}O_{3}+Ni\;\;\;\;\;}}{900��1000��}$CO+3H2
��4����ҵ�Ϻϳɰ��������в���400��500��ĸ��£�ԭ��֮һ�ǿ��Ǵ����Ļ��ԣ������Ϊ����߷�Ӧ���ʣ����̴ﵽƽ���ʱ�䣻����30��50MPa��ѹǿ����Ϊ�˱�֤�ϸߵķ�Ӧ���ʺͽϸߵIJ����Լ��豸����ѹ�̶������ǵģ��ڸ������´����Ļ������Ӧ���ʿ죬ͬʱԭ�ϵ�ת���ʽϸߣ�
�ʴ�Ϊ��B
��5�����ݻ�ѧ����ʽ���Ȼ����봿������2NaCl��Na2CO3
2��58.5 106
11.7t��10.6t�����Ȼ��Ƴ������ʱ���ɴ���10.6t����ʵ�����ɴ���10.07t���Ȼ��Ƶ�������Ϊ��$\frac{10.07}{10.6}$��100%=95%��
�ʴ�Ϊ��95%��
���� ������һ���йع�ҵ�ƴ���֪ʶ��һ���ۺ�ʵ����Ŀ������ѧ�������ͽ����������������պ����Ƽ��ԭ���ǽ���Ĺؼ�����Ŀ�Ѷ��е�

| A�� | ���ʵ�λ�Ƶ�һ������������ | B�� | ��ʾ���������ĵ�λ | ||
| C�� | ���ʵ����ĵ�λ | D�� | ��ʾ���������ĵ�λ |
 ��ͼ��ʾ��ʵ������Ũ���ᡢ�廯�ƣ�������HBr�����Ҵ���Ӧ���Ʊ������飨C2H5Br����װ�ã���Ӧ��Ҫ���ȣ�ͼ��ʡȥ�˼г������װ�ã��й����ʵ��������±���
��ͼ��ʾ��ʵ������Ũ���ᡢ�廯�ƣ�������HBr�����Ҵ���Ӧ���Ʊ������飨C2H5Br����װ�ã���Ӧ��Ҫ���ȣ�ͼ��ʡȥ�˼г������װ�ã��й����ʵ��������±���| �Ҵ� | ������ | �� | |
| ͨ�������״̬ | ��ɫҺ�� | ��ɫҺ�� | �����ɫҺ�� |
| �ܶ�/g•cm-3 | 0.79 | 1.44 | 3.1 |
| �е�/�� | 78.5 | 38.4 | 59 |
��2����ȴ��Ӧ��h���h����i�����ڽ���������C�У��Ʊ������У������Ũ�����������ʵ�ϡ�ͣ���Ŀ�ģ���ԭ����b������ĸ����
a��ˮ�Ƿ�Ӧ�Ĵ�������b������Br2������ ����c������HBr�Ļӷ�
��3�����ȵ�Ŀ���Ǽӿ췴Ӧ������ʹ������������ʹ��D������ȴ��ԭ����ʹ������Һ�������ռ���
��4��A��Һ�����������ɫ������֣�д���������Ļ�ѧ����ʽ2HBr+H2SO4��Ũ��$\stackrel{��}{��}$Br2��+2H2O+SO2����д��A������������Ļ�ѧ����ʽHBr+CH3CH2OH$\stackrel{��}{��}$CH3CH2Br+H2O��
��5����E�в�Ʒת�뵽��Һ©���У�����©���м�������Na2SO3��Һ�����ú��Һ������Na2SO3��Һ��Ŀ���dz�ȥ����������ʣ���ҺʱĿ������뿪©���ķ�ʽ�Ǵ�����ĵ����ų�����
| A�� | ��ԭ��صĸ����͵��ص������϶�����ʧ���ӵ�������Ӧ | |
| B�� | �ö��Ե缫���Na2SO4��Һ������������������ʵ���֮��Ϊ1��2 | |
| C�� | �ö��Ե缫��ⱥ��NaCl��Һ������0.1 mol����ת�ƣ�������0.1 mol NaOH | |
| D�� | �Ʋ������п����ȶ����������ʴ |
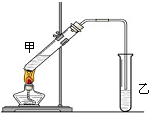 ��˵�����¾��ϴ��ر��㡱����ԭ���Ǿ��ڴ������������������ζ��������������ʵ����������Ҳ��������ͼ��ʾ��װ����ģ��ù��̣���ش��������⣺
��˵�����¾��ϴ��ر��㡱����ԭ���Ǿ��ڴ������������������ζ��������������ʵ����������Ҳ��������ͼ��ʾ��װ����ģ��ù��̣���ش��������⣺ CH3COOC2H5+H2O��CH3COOH+Na2CO3=NaHCO3+CH3COONa��
CH3COOC2H5+H2O��CH3COOH+Na2CO3=NaHCO3+CH3COONa��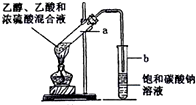 ��ͼΪʵ������ȡ����������װ�ã�
��ͼΪʵ������ȡ����������װ�ã� CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O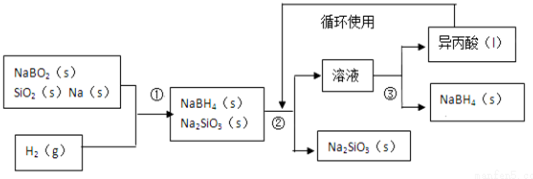
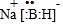 ������BԪ�صĻ��ϼ�Ϊ+3
������BԪ�صĻ��ϼ�Ϊ+3