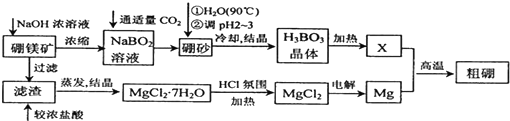
��Ŀ����
9����ҵ�÷���м��ԭ���̿���Ҫ�ɷ�ΪMnO2����������������SiO2�ȣ��Ʊ������̣��乤������ʾ����ͼ��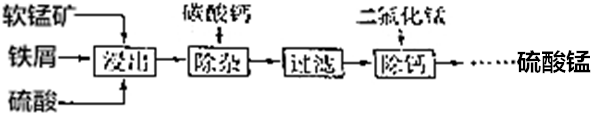
��1�����������У�MnO2����м��ԭΪMn2+������Һ���ò������漰����Ҫ���ӷ�Ӧ�У�Fe+2H+�TFe2++H2����MnO2+2Fe2++4H+�TMn2++2Fe3++2H2O��2Fe3++Fe=3Fe2+��
��2�����ӹ����м���̼��Ƶ�Ŀ���ǵ�����Һ��pH����Fe3+ת��ΪFe��OH��3����������ȥ��
��3�����ƹ����У���ʹCa2+������ȫ[����Һ��c��Ca2+����10-5mol•L-1ʱ��������ΪCa2+�ѳ�����ȫ]������Һ��c��F-��Ӧ��1��10-3mol•L-1������֪�ó�����CaF2��Ksp=1��10-11����
��4�������������У�������������˹��࣬��ԭ���DZ�������CaSO4������
��5����ҵ�Ͽ��õ�������̺���������Һ�ķ����Ʊ��������̣�Mn2+������ʧ����ת��ΪMnO2��
���� ��1�������ܹ������ᷴӦ���������������������ݴ�д����Ӧ�����ӷ���ʽ��
��2������Һ�д���ˮ��ƽ�⣺Fe3++3H2O?Fe��OH��3+3H+������CaCO3������CaCO3��s��?Ca2+��aq��+CO32-��aq����CO32-+2H+�TCO2��+H2O����Һ��������Ũ�ȼ�С��ʹ��Fe3+��ˮ��ƽ��������Ӧ�����ƶ�����Fe3+ת��ΪFe��OH��3������
��3��Ksp��CaF2��=1��10-11=c��Ca2+����c2��F-��������Һ��c��Ca2+����10-5mol•L-1ʱ������ΪCa2+�ѳ�����ȫ����c��F-����$\sqrt{\frac{1��1{0}^{-11}}{1{0}^{-5}}}$mol/L��
��4��������������������ܹ���Ӧ������������ƣ�
��� �⣺��1�������ܹ������ᷴӦ����������������������Ӧ�����ӷ���ʽΪ��Fe+2H+�TFe2++H2����
�ʴ�Ϊ��Fe+2H+�TFe2++H2����
��2������Һ�д���ˮ��ƽ�⣺Fe3++3H2O?Fe��OH��3+3H+������CaCO3������CaCO3��s��?Ca2+��aq��+CO32-��aq����CO32-+2H+�TCO2��+H2O����Һ��������Ũ�ȼ�С��ʹ��Fe3+��ˮ��ƽ��������Ӧ�����ƶ������Fe3+ת��ΪFe��OH��3����������ȥ��
�ʴ�Ϊ��������Һ��pH����Fe3+ת��ΪFe��OH��3����������ȥ��
��3��Ksp��CaF2��=1��10-11=c��Ca2+����c2��F-��������Һ��c��Ca2+����10-5mol•L-1ʱ������ΪCa2+�ѳ�����ȫ����c��F-����$\sqrt{\frac{1��1{0}^{-11}}{1{0}^{-5}}}$mol/L=1��10-3mol•L-1��
�ʴ�Ϊ����1��10-3mol•L-1��
��4��������������������ܹ���Ӧ������������ƣ��������������࣬��Һ��������Ƴ�����
�ʴ�Ϊ����������CaSO4������
���� ���⿼�����Ʊ���������ƣ���Ŀ�Ѷ��еȣ���ȷ�Ʊ����̼�����ԭ��Ϊ���ؼ���ע�������������ܽ�ƽ�⼰�ܶȻ��ļ��㷽��������������ѧ���ķ�����������ѧ���㡢��ѧʵ��������

 ͬ����ϰ���ϴ�ѧ������ϵ�д�
ͬ����ϰ���ϴ�ѧ������ϵ�д� ͬ����ϰ���ϴ�ѧ������ϵ�д�
ͬ����ϰ���ϴ�ѧ������ϵ�д� ����ϰ�⽭��ϵ�д�
����ϰ�⽭��ϵ�д� ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�| A�� | �ȵĴ�����Һȥ������ǿ | |
| B�� | ���ᱵ������李���ˮ����������� | |
| C�� | ��Na2S��Һ��AlCl3��Һ�����ȡAl2S3 | |
| D�� | ̼�ᱵ�����ᱵ�������������� |
| A�� | c��CH3COOH����Ϊԭ����$\frac{1}{10}$ | B�� | $\frac{c��C{H}_{3}CO{O}^{-}��}{c��C{H}_{3}COOH��}$�ı�ֵ���� | ||
| C�� | c��H+����Ϊԭ����$\frac{1}{10}$ | D�� | ��Һ�ĵ�������ǿ |
| A�� | 0.2molCuSO4 | B�� | 0.2molCuO | C�� | 0.2molCu��OH��2 | D�� | 0.2molCuCO3 |