��Ŀ����
12��ʵ��������0.1mol•L-1��BaCl2��Һ�ɷ������ν��У���һ�Σ���������ƽ��ȡ5.2g��ˮBaCl2���壮�ڶ��Σ��ܽ⾧������0.1mol•L-1��BaCl2��Һ����һ�β��������¼�����A�������벦��0.2g����B�������벦����0������C������ƽ���ߵ������ϸ���һ�Ÿɾ��ĵ���������ֽ��������ƽ���ߵ���ĸʹ��ƽƽ�⣻D��ȡ��ҩƷ��������Ż�������ڣ�E���������������Ӿ�������ƽƽ�⣻F���������Ϸ���5g���룮��1������ȷ�IJ���˳���ǣ�����ţ���B��C��F��A��E��D��B
��2����E�����У�ֻ������������ʱ�������������������ҩ�ף���������������������С����ҩ��ʹʹ��������������ֽ�ϣ�
��3���ڶ��β�����Ӧ�Ƚ�5.2g BaCl2����������ˮ�ܽ⣬�ܽ������ʹ�õ���Ҫ�������ձ�����������Ȼ����Һת��250mL����ƿ�У��پ�ϴ�ӡ����ݡ�ҡ�Ⱥɵõ�0.1mol•L-1 BaCl2��Һ��
��4�����в���ʹ���Ƶ�BaCl2��ҺŨ��ƫС����AC������ţ���
A����������������ϣ�BaCl2���������Ͻ��г���
B��ѡ�õ�����ƿ������������ˮ
C������ҡ�Ⱥ�Һ���½����ּ�ˮ���̶���
D���������ƹ����У�����ƿ����
���� ��1��ʹ��������ƽ�IJ���Ϊ�������������������������Ʒ��ȡ��Ʒ��ȡ�����������㣻
��2�����ݳ�������ҩƷʱ������ҩƷ����ȷ�������
��3����������һ�����ʵ���Ũ����Һ�IJ������輰ÿ���õ����������
��4�������������������ʵ����ʵ�������Һ�������Ӱ�죬����C=$\frac{n}{V}$������������
��� �⣺��1��������ƽ�IJ���Ϊ�������������������������Ʒ��ȡ��Ʒ��ȡ�����������㣬����ȷ�IJ���˳���ǣ�B��C��F��A��E��D��B��
�ʴ�Ϊ��C��F��E��D��B��
��2����������ҩƷʱ������ҩƷֻȱ��������ʱ��������ҩ�ף���������������������С����ҩ��ʹʹ��������������ֽ�ϣ�
�ʴ�Ϊ��������ҩ�ף���������������������С����ҩ��ʹʹ��������������ֽ�ϣ�
��3������һ�����ʵ���Ũ����Һ�IJ������裺���㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȣ��ܽ�ʱ������Ӧ�����ձ��У��ò��������Ͻ��裻
�ʴ�Ϊ���ձ�����������250mL����ƿ��ϴ�ӡ����ݡ�ҡ�ȣ�
��4��A����������������ϣ�BaCl2���������Ͻ��г��������³�ȡ������ƫС�����ʵ����ʵ���ƫС����Һ��Ũ��ƫ�ͣ���Aѡ��
B��ѡ�õ�����ƿ������������ˮ�������ʵ����ʵ�������Һ��������������Ӱ�죬��Һ��Ũ�Ȳ���Ӱ�죬��B��ѡ��
C������ҡ�Ⱥ�Һ���½����ּ�ˮ���̶��ߣ�������Һ�����ƫ����Һ��Ũ��ƫ�ͣ���Cѡ��
D���������ƹ����У�����ƿ�����ᵼ����Һ��ϲ����ȣ���Һ���������ƫ���ƫС����Һ��Ũ�ȿ���ƫ�ͻ�ƫ�ߣ���D��ѡ��
��ѡ��AC��
���� ���⿼��������һ�����ʵ���Ũ����Һ�����ؿ���ѧ����ʵ��������������ճ̶ȣ���ȷʵ��ԭ���ͻ��������ǽ���ؼ�����Ŀ�ѶȲ���ע��������ƽ��ʹ�÷�����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�2H2��g��+O2��g���T2H2O��g����H2=-Q2 KJ/mol
2H2��g��+O2��g���T2H2O��1����H3=-Q3KJ/mol
�����£�ȡ�����Ϊ4��1�ļ���������Ļ������11.2L�����ۺϳɱ�״����������ȫȼ�պ�ָ������£�������˵����ȷ���ǣ�������
| A�� | �ų�������Ϊ��0.4Q1+0.05Q3��KJ | B�� | �ų�������Ϊ��0.4Q1+0.05Q2��KJ | ||
| C�� | ��H2=��H3 | D�� | ��H2����H3 |
| A�� | Ԫ��R λ�����ڱ���IB �壬��ԭ������Ϊa����ԭ������Ϊa-3 ��Ԫ��λ�ڢ�B �� | |
| B�� | ��Ԫ�����ڱ��� 114 ��Ԫ�ص���һ����ͬһ��Ԫ�ص�ԭ�������� 82 | |
| C�� | ������ͬ���Ӳ�ṹ������Ԫ������ΪX2+��Y+��������������ˮ����ļ���X��Y | |
| D�� | �����ڱ��н�����ǽ����ķֽ��ߴ������ҵ����������¡���ʴ�ĺϽ���� |
| A�� | �ס��졢�� | B�� | �졢�ڡ��� | C�� | �졢�졢�� | D�� | �ס��ڡ��� |
CH2=CHCOOH+HOCH3��CH2=CHCOOCH3+H2O
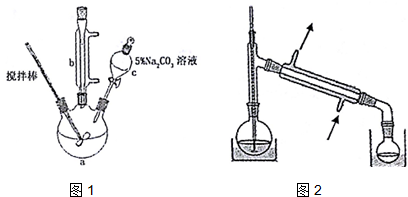
��ȡ10.0g��ϩ���6.0g�״�������������ƿ�У����Ӻ������ܣ��ý�������裬ˮԡ���ȣ�
�ڳ�ַ�Ӧ����ȴ������Һ�м���5% Na2CO3��Һϴ�����ԣ�
�۷�Һ��ȡ�ϲ���״Һ�壬������ˮNa2SO4����������ռ�70-90����֣�
�����õ�����Ϣ��
| �е� | �ܽ��� | ||
| ��ϩ�� | 141�� | ��ˮ���ܣ��������л��ܼ� | �ж� |
| �״� | 65�� | ��ˮ���ܣ��������л��ܼ� | �ӷ����ж� |
| ��ϩ����� | 80.5�� | ������ˮ���������л��ܼ� | �ӷ� |
��1������c�������Ƿ�Һ©����
��2�����Һ��5%0Na2CO3��Һϴ�ӵ�Ŀ���dz�ȥ���Һ�еı�ϩ��ͼ״������ͱ�ϩ��������ܽ�ȣ���
��3����д������100g 5% Na2CO3��Һ��ʹ�õIJ��������ձ�������������Ͳ��
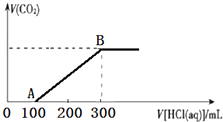
��4�����ڲ�Ʒ������������г�װ��δ��������ͼ2����2��������ֱ�д���¶ȼ�ˮ����λ�á�β�ӹ�����ƿ�ӿ��ܷ⣮
Ϊ������ʣ��������ʵ�飺
�ٽ���״�����ᴿ��ƽ���ֳ�5�ݣ�ȡ��1��������ƿ�У�����2.5mol/L��KOH��Һ10-00mL������ʹ֮��ȫˮ�⣮
���÷�̪��ָʾ��������ȴ�����Һ�еμ�0.5mol/L��HCI��Һ���к�����KOH���ε��յ�ʱ����������20.00mL��
��5�����㱾��������Ӧ��ϩ���ת����54.0%��
��6�����о�2����ʵ������Ҫ��ȡ�İ�ȫ������ʩͨ�����ʵ�顢��ֹ����
| A�� | 5mL | B�� | 20mL | C�� | ����5mL | D�� | ��5mL |
 200mLij���ʵ���Ũ�ȵ�NaOH��Һ�л���ͨ��һ������CO2����ַ�Ӧ�õ�Na2CO3��NaHCO3�Ļ����Һ��������������Һ�У���εμ�2mol•L-1�����ᣬ����������������������������ϵ��ͼ��ʾ��
200mLij���ʵ���Ũ�ȵ�NaOH��Һ�л���ͨ��һ������CO2����ַ�Ӧ�õ�Na2CO3��NaHCO3�Ļ����Һ��������������Һ�У���εμ�2mol•L-1�����ᣬ����������������������������ϵ��ͼ��ʾ��

 ��
�� ��
�� ��
��