��Ŀ����
 ij�о���ѧϰС�����о���������Ư������ʱ���ӡ�������Ư������ʵ������������ˮ��Ӧ���ɵĴ������Ư�����á��õ�������Ϊ��̽����������Ư�����õ����Ƕ������������Ƕ���������ˮ���õIJ����С�����������ʵ�飮��ش�������⣮
ij�о���ѧϰС�����о���������Ư������ʱ���ӡ�������Ư������ʵ������������ˮ��Ӧ���ɵĴ������Ư�����á��õ�������Ϊ��̽����������Ư�����õ����Ƕ������������Ƕ���������ˮ���õIJ����С�����������ʵ�飮��ش�������⣮��1��ʵ�������������Ʒ�ĩ��������ȡ��������������������������Һ��Ӧѡ��
A��98%Ũ���ᡡ��������B��70%���ᡡ��������C��10%ϡ����
��2��Ϊ��̽��SO2�ܷ�ʹƷ����ɫ����ͬѧѡ������ȷ��ҩƷ�����������ͼ��ʾʵ��װ�ã���ָ��ʵ��װ������еIJ�����֮������
��3����ͬѧѡ������ȷװ�ú�ʵ���п��ƶ��������Դ�Լÿ��3�����ݵ��ٶ�ͨ��Ʒ��ľƾ���Һʱ������һСʱ��Ʒ���Բ���ɫ��Ϊ�ˣ�����ΪʹƷ���ˮ��Һ��ɫ����������
���㣺̽������������ˮ��Ʒ����Һ�ķ�Ӧ,��������Ļ�ѧ����
ר�⣺ʵ�������
��������1������Ũ����Ͷ�����������ʷ�����Ũ�ȴ�ʱ�����Է�����ʽ���ڣ�����ϡʱ����������������ˮ���ݴ˷�����
��2������ʵ��Ŀ�ļ�������������ʷ�����Ӧ��ȡ����Ķ����������壬Ϊ����Һ�������Ӧѡȡ����������������
��3�����ݶ�������Ʒ����Һ��Ʒ��ƾ���Һ�����ʷ�����
��2������ʵ��Ŀ�ļ�������������ʷ�����Ӧ��ȡ����Ķ����������壬Ϊ����Һ�������Ӧѡȡ����������������
��3�����ݶ�������Ʒ����Һ��Ʒ��ƾ���Һ�����ʷ�����
���
�⣺��1��������������ƹ�����ȡ�����������������ӷ�Ӧ���������Ũ�ȹ����������Է�����ʽ���ڣ�û�е����H+�����ܷ�Ӧ����SO2���������Ũ�ȹ��ͣ���������������ˮ�������ڶ�������ų�������ѡȡ70%���ᣬ
�ʴ�Ϊ��B�� 98%Ũ������c��H+��Ũ��̫С��10%������Һ��ˮ�����ߣ�����ʹSO2�ų���
��2��Ϊ�������������Ӧѡȡ��Һ©������ʵ����̽�������SO2�ܲ���ʹƷ����ɫ�����Զ�������ͨ��Ʒ����ҺǰҪ���и��
�ʴ�Ϊ����ȱ��SO2�ĸ���װ�ã��ڲ����ó���©����Ӧ�÷�Һ©����
��3�����������ˮ��Ӧ���������ᣬ��������;ƾ�����Ӧ������������ʹƷ����Һ��ɫ������ʹƷ��ľƾ���Һ��ɫ���ɴ˵ó�S02��Ư��������SO2��ˮ���õIJ��ﵼ�µģ����������ˮ��Ӧ���������ᡢ�������������������������ӡ�������������ӵ�����������������ӣ�����ʹƷ���ˮ��Һ��ɫ���������ǣ�HSO3-��SO32-��H2SO3��
�ʴ�Ϊ��HSO3-��SO32-��H2SO3��
�ʴ�Ϊ��B�� 98%Ũ������c��H+��Ũ��̫С��10%������Һ��ˮ�����ߣ�����ʹSO2�ų���
��2��Ϊ�������������Ӧѡȡ��Һ©������ʵ����̽�������SO2�ܲ���ʹƷ����ɫ�����Զ�������ͨ��Ʒ����ҺǰҪ���и��
�ʴ�Ϊ����ȱ��SO2�ĸ���װ�ã��ڲ����ó���©����Ӧ�÷�Һ©����
��3�����������ˮ��Ӧ���������ᣬ��������;ƾ�����Ӧ������������ʹƷ����Һ��ɫ������ʹƷ��ľƾ���Һ��ɫ���ɴ˵ó�S02��Ư��������SO2��ˮ���õIJ��ﵼ�µģ����������ˮ��Ӧ���������ᡢ�������������������������ӡ�������������ӵ�����������������ӣ�����ʹƷ���ˮ��Һ��ɫ���������ǣ�HSO3-��SO32-��H2SO3��
�ʴ�Ϊ��HSO3-��SO32-��H2SO3��
���������⿼����̽����������Ư���Ե�ʵ�飬��ȷ�����ʵ������ǽⱾ��ؼ���ע����öԱȷ������з������Ѷ��еȣ�

��ϰ��ϵ�д�
 ���100�ֵ�Ԫ�Ż�������ϵ�д�
���100�ֵ�Ԫ�Ż�������ϵ�д�
�����Ŀ
���з���������ԭ�Ӷ����������8���ӽṹ���ǣ�������
| A��������COCl2�� |
| B�����Ȼ��ף�PCl5�� |
| C����������CF2Cl2�� |
| D����������BF3�� |
�������л�����ص�������ȷ���ǣ�������
| A��CH4O��C2H6Oһ����Ϊͬϵ�� |
| B��1mol C6H6�������к���3mol̼̼˫�� |
| C����Ũ�����뵰���ʵ���ɫ��Ӧ���ֵ����� |
| D�������������м���NaOH ��Һ������Һ�����ȥ���������е��������� |
����˵������ȷ���ǣ�������
| A����NaHCO3��Һ���У�C��CO32-��+C��OH-��=C��H+��+C��H2CO3�� |
| B��FeCl3��Һ�д��ڣ�Fe3++3H2O?Fe��OH��3+3H+ ��ˮ��ƽ�⣬������Һ�������HCl��NaOH��Һ������ʹ��ƽ�������ƶ� |
| C��������������ȡʵ���У��Լ������˳���ǣ���ˮ�Ҵ���Ũ����������� |
| D��ʵ������ȡ�����ԭ���ǣ���ˮ�����ƺͼ�ʯ�ҹ��ȣ����м�ʯ�ҵijɷ���NaOH��CaO |
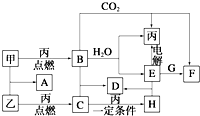 �ס��ҡ���Ϊ�������ʣ�A��B��C��D��E��F��G��H��Ϊ��ѧ��ѧ�г����Ļ��������B��G����ɫ��Ӧ��Ϊ��ɫ��C��ʹƷ����Һ��ɫ����һ�������£��������ת����ϵ��ͼ��ʾ����ش��������⣺
�ס��ҡ���Ϊ�������ʣ�A��B��C��D��E��F��G��H��Ϊ��ѧ��ѧ�г����Ļ��������B��G����ɫ��Ӧ��Ϊ��ɫ��C��ʹƷ����Һ��ɫ����һ�������£��������ת����ϵ��ͼ��ʾ����ش��������⣺ FeCl3��ͭ��ӡˢ������ӡˢ��·ͭ�帯ʴ������Ҫ��ش��������⣺
FeCl3��ͭ��ӡˢ������ӡˢ��·ͭ�帯ʴ������Ҫ��ش��������⣺