��Ŀ����
��ҵ��Ϊ�˴�����Cr2O72-�����Է�ˮ����������Ĵ�������������ҵ��ˮ�����������NaCl����FeΪ�缫���е�⣬����һ��ʱ�����Cr��OH��3��Fe��OH��3�������ɣ���ҵ��ˮ��Cr3+��Ũ��������10-5mol/L�ɷ����ŷű����ش��������⣺
��1�����ʱ�����Ϸ����ĵ缫��Ӧʽ�� ��
��2��д��Cr2O72-��ΪCr3+�����ӷ���ʽ ��
��3����ҵ��ˮ�����Ա�Ϊ���Ե�ԭ���� ��
��4����֪������Cr��OH��3��KSP=1��10-32������¶��·����ŷű�����Һ�����pHΪ ��
��1�����ʱ�����Ϸ����ĵ缫��Ӧʽ��
��2��д��Cr2O72-��ΪCr3+�����ӷ���ʽ
��3����ҵ��ˮ�����Ա�Ϊ���Ե�ԭ����
��4����֪������Cr��OH��3��KSP=1��10-32������¶��·����ŷű�����Һ�����pHΪ
���㣺ԭ��غ͵��صĹ���ԭ��
ר�⣺�绯ѧר��
��������1��FeΪ�缫���е��ʱ����������ʧ���ӷ���������Ӧ�������������ӷŵ�����������ͬʱ�����������������������ɣ�
��2�����������£�Cr2O72-���������ӷ���������ԭ��Ӧ�����������Ӻ����ӣ�
��3�������ϼ�����������ԭ�����������ӣ�
��4�������ܶȻ������Լ�ˮ�����ӻ����������м��㣮
��2�����������£�Cr2O72-���������ӷ���������ԭ��Ӧ�����������Ӻ����ӣ�
��3�������ϼ�����������ԭ�����������ӣ�
��4�������ܶȻ������Լ�ˮ�����ӻ����������м��㣮
���
�⣺��1��FeΪ�缫���е��ʱ�������ǻ��õ缫����缫��������ʧ���ӵ�������Ӧ����Fe-2e-�TFe2+���ʴ�Ϊ��Fe-2e-�TFe2+��
��2��Cr2O72-����ǿ�����ԣ����Խ�������������Ϊ�����ӣ���������ԭΪ2Cr3+����Ӧ��ʵ���ǣ�6Fe2++Cr2O72-+14H+�T6Fe3++2Cr3++7H2O���ʴ�Ϊ��6Fe2++Cr2O72-+14H+�T6Fe3++2Cr3++7H2O��
��3��������Ӧ����H+����Һ�е�������ԭ��ӦҲ����H+��������Һ��������Ũ�Ƚ��ͣ��Ӷ�ʹ��ҺpH���ߣ�
�ʴ�Ϊ��������H+�ŵ磬Cr2O72-��Fe2+��Ӧ��������H+��ʹˮ�ĵ���ƽ�������ƶ���ʹ��ҺpH���ߣ�
��4����c��Cr3+��=10-5mol/Lʱ����Һ��c��OH-��=
=10-9 mol/L��
c��H+���T
=10-5mol/L��pH=5��
��Ҫʹc��Cr3+������10-5mol/L����Һ��pHӦ����5��
�ʴ�Ϊ��5��
��2��Cr2O72-����ǿ�����ԣ����Խ�������������Ϊ�����ӣ���������ԭΪ2Cr3+����Ӧ��ʵ���ǣ�6Fe2++Cr2O72-+14H+�T6Fe3++2Cr3++7H2O���ʴ�Ϊ��6Fe2++Cr2O72-+14H+�T6Fe3++2Cr3++7H2O��
��3��������Ӧ����H+����Һ�е�������ԭ��ӦҲ����H+��������Һ��������Ũ�Ƚ��ͣ��Ӷ�ʹ��ҺpH���ߣ�
�ʴ�Ϊ��������H+�ŵ磬Cr2O72-��Fe2+��Ӧ��������H+��ʹˮ�ĵ���ƽ�������ƶ���ʹ��ҺpH���ߣ�
��4����c��Cr3+��=10-5mol/Lʱ����Һ��c��OH-��=
| 3 |
| ||
c��H+���T
| 10-14 |
| 10-9 |
��Ҫʹc��Cr3+������10-5mol/L����Һ��pHӦ����5��
�ʴ�Ϊ��5��
���������⿼���˵��ԭ�����������ܶȻ��ļ��㡢��Ŀ�ѶȽϴ��漰��֪ʶ��϶࣬ע�����յ��ԭ�����������ܶȻ��ĸ�����㷽��������������ѧ���ķ�����������������ѧ����������

��ϰ��ϵ�д�
 ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д�
�����Ŀ
���и�������ʾ��ͼһ�µ��ǣ�������
A�� ��ʾ��Mg2+��Al3+��NH4+������Һ�еμ�NaOH��Һʱ������������NaOH������Ĺ�ϵͼ�����������ӵ����ʵ���֮��Ϊ��n��Mg2+����n��Al3+����n�� NH4+��=2��1��2������ʹ�õ�NaOH��Ũ��Ϊ2mol?L-1�� |
B�� �����߱�ʾij��Ӧ���̵������仯��������A��g����B��g����Ӧ��������C��g��ʱ����H��0����ʹ����������Eֵ���С�� |
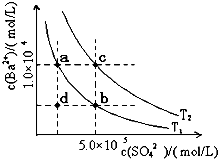
C�� ��������ʾ�������ữ��MgSO4��Һ�м���Ba��OH��2��Һʱ���������������ʵ�����n�������Ba��OH��2��Һ�����V��֮��Ĺ�ϵͼ |
D�� ��ʾ��һ��������������Һ�еμ�Ba��OH��2��Һʱ���������������ʵ�����n�������Ba��OH��2��Һ�����V��֮��Ĺ�ϵͼ |
������Һ�������ɺ����գ����������ʹ�����ǣ�������
| A��AlCl3 |
| B��KHCO3 |
| C��Fe2��SO4��3 |
| D��NH4HCO3 |
����ʵ��������������ȷ���ǣ�������
| A�����ڿ�����ȼ�ղ�����ɫ���棬���ɵ���ɫ�������� |
| B��������ˮ��dz����ɫ����ˮ���������pH����� |
| C������ϡ���ᷴӦ�����Ȼ�������������������Ӧ���������Ȼ��� |
| D��Ư�۵���Ч�ɷ��Ǵ�����ƣ�������Ư��������Ϊ��ˮ��Һ����������ǿ�����ԵĴ����� |


 �ش��������⣺
�ش��������⣺ ��1����ͼ��ʾ������������ͨ��ʢ�и�����ɫ�����Ĺ��ƿ��ʢ�г�ʪ��ɫ�����Ĺ��ƿ���ɹ۲쵽��������
��1����ͼ��ʾ������������ͨ��ʢ�и�����ɫ�����Ĺ��ƿ��ʢ�г�ʪ��ɫ�����Ĺ��ƿ���ɹ۲쵽��������