��Ŀ����
����Ԫ��X��Y��Z��W��ԭ���������������������Ϣ���±���
��1��Yλ��Ԫ�����ڱ��� ���� �壬���̬ԭ��δ�ɶԵ����� ����
��2��X�ĵ縺�Ա�W�� �����С������Y�������̬�⻯���X�������̬�⻯����Һ��������Ҫԭ���� ��
��3��Z��ͬ�����������ڵ�����Ԫ�ص�ԭ����Ƚϣ����ߵ�һ�������ɴ�С��˳��Ϊ ����Ԫ�ط��ű�ʾ����Y��Z�γɵĻ�����Ϊ ���壬��������ˮǿ��ˮ��Ļ�ѧ����ʽΪ ��
��4����һ���¶��£���һ���ݻ�������ܱ������г���1molY2��3mol������������Ӧ��Y2��g��+3H2��g��?2YH3��g����H=-akJ/mol���ڸ������´ﵽƽ��ʱ�ų�������ΪbkJ����ƽ�ⳣ������ʽK= ������ʼ��������г���2molYH3������ͬ�¶��´ﵽƽ��ʱ����Ӧ���������յ�����ΪckJ����a��b��c����֮��Ĺ�ϵΪ ����һ��ʽ�ӱ�ʾ����
| Ԫ�� | �����Ϣ |
| X | X�Ļ�̬ԭ�Ӻ���ֻ�������ܼ����Ҹ��ܼ���������� |
| Y | Y�Ļ�̬ԭ�����������������ڲ����������2.5�� |
| Z | Z�Ļ�̬�۵��ӽṹΪnsn-1 |
| W | W���ʳ��ڻ�ɽ�ڸ��������֣���������������������Ҫԭ��֮һ |
��2��X�ĵ縺�Ա�W��
��3��Z��ͬ�����������ڵ�����Ԫ�ص�ԭ����Ƚϣ����ߵ�һ�������ɴ�С��˳��Ϊ
��4����һ���¶��£���һ���ݻ�������ܱ������г���1molY2��3mol������������Ӧ��Y2��g��+3H2��g��?2YH3��g����H=-akJ/mol���ڸ������´ﵽƽ��ʱ�ų�������ΪbkJ����ƽ�ⳣ������ʽK=
���㣺λ�ýṹ���ʵ����ϵӦ��
ר�⣺Ԫ����������Ԫ�����ڱ�ר��
����������Ԫ��X��Y��Z��W��ԭ��������������X�Ļ�̬ԭ�Ӻ���ֻ�������ܼ����Ҹ��ܼ���������ȣ���Xԭ�Ӻ�������Ų�Ϊ1s22s22p2����XΪCԪ�أ�
Y�Ļ�̬ԭ�����������������ڲ����������2.5������Yԭ�Ӻ�����2�����Ӳ㣬����������Ϊ5����YΪNԪ�أ�
Z�Ļ�̬ԭ�Ӽ۵����Ų�Ϊnsn-1��ԭ������������Ԫ�أ���n=3��Z�Ļ�̬ԭ�Ӽ۵����Ų�Ϊ3s2����ZΪMgԪ�أ�
W���ʳ��ڻ�ɽ�ڸ��������֣���������������������Ҫԭ��֮һ����WΪSԪ�أ�
��1��YΪNԪ�أ�
��2��XΪCԪ�أ�WΪSԪ�أ��ǽ�����S��ǿ��Y�������̬�⻯��ΪNH3�����Ӽ����γ������
��3��ͬ�����������Ԫ�ص�һ�����ܳ��������ƣ�����IIA����IIIA�壬��VA����VIA�巴����Y��Z�γɵĻ�����ΪMg3N2��Mg3N2��ˮ��Ӧ����������þ�Ͱ�����
��4����֪Y2��g��+3H2��g��?2YH3��g����H=-akJ/mol������ƽ�ⳣ���ĺ�����дK�ı���ʽ���������г���2molNH3���������г���1molN2��3molH2Ϊ��Чƽ�⣬����ƽ��ʱ����ֵ�����Ӧ��ȣ���ƽ��ʱ���������ʵ���Ϊymol�������Ȼ�ѧ����ʽ���a��b���ݴ˽��
Y�Ļ�̬ԭ�����������������ڲ����������2.5������Yԭ�Ӻ�����2�����Ӳ㣬����������Ϊ5����YΪNԪ�أ�
Z�Ļ�̬ԭ�Ӽ۵����Ų�Ϊnsn-1��ԭ������������Ԫ�أ���n=3��Z�Ļ�̬ԭ�Ӽ۵����Ų�Ϊ3s2����ZΪMgԪ�أ�
W���ʳ��ڻ�ɽ�ڸ��������֣���������������������Ҫԭ��֮һ����WΪSԪ�أ�
��1��YΪNԪ�أ�
��2��XΪCԪ�أ�WΪSԪ�أ��ǽ�����S��ǿ��Y�������̬�⻯��ΪNH3�����Ӽ����γ������
��3��ͬ�����������Ԫ�ص�һ�����ܳ��������ƣ�����IIA����IIIA�壬��VA����VIA�巴����Y��Z�γɵĻ�����ΪMg3N2��Mg3N2��ˮ��Ӧ����������þ�Ͱ�����
��4����֪Y2��g��+3H2��g��?2YH3��g����H=-akJ/mol������ƽ�ⳣ���ĺ�����дK�ı���ʽ���������г���2molNH3���������г���1molN2��3molH2Ϊ��Чƽ�⣬����ƽ��ʱ����ֵ�����Ӧ��ȣ���ƽ��ʱ���������ʵ���Ϊymol�������Ȼ�ѧ����ʽ���a��b���ݴ˽��
���
�⣺����Ԫ��X��Y��Z��W��ԭ��������������X�Ļ�̬ԭ�Ӻ���ֻ�������ܼ����Ҹ��ܼ���������ȣ���Xԭ�Ӻ�������Ų�Ϊ1s22s22p2����XΪCԪ�أ�
Y�Ļ�̬ԭ�����������������ڲ����������2.5������Yԭ�Ӻ�����2�����Ӳ㣬����������Ϊ5����YΪNԪ�أ�
Z�Ļ�̬ԭ�Ӽ۵����Ų�Ϊnsn-1��ԭ������������Ԫ�أ���n=3��Z�Ļ�̬ԭ�Ӽ۵����Ų�Ϊ3s2����ZΪMgԪ�أ�
W���ʳ��ڻ�ɽ�ڸ��������֣���������������������Ҫԭ��֮һ����WΪSԪ�أ�
��1��YΪNԪ�أ��ڵڶ����ڣ���VA�壻NԪ��ԭ�ӵ�2p�������3�������ӣ���δ�ɶԵ�����Ϊ3��
�ʴ�Ϊ������VA��3��
��2��XΪCԪ�أ�WΪSԪ�أ��ǽ�����S��ǿ����X�ĵ縺�Ա�W��С��Y�������̬�⻯��ΪNH3�����Ӽ����γ������CH4�ķ��Ӽ䲻���γ����������Y�������̬�⻯���X�������̬�⻯����Һ����
�ʴ�Ϊ��С��������֮������γ������
��3��ͬ�����������Ԫ�ص�һ�����ܳ��������ƣ�����IIA����IIIA�壬��VA����VIA�巴�������һ�����ܵĴ�С��ϵΪ��Mg��Al��Na��Y��Z�γɵĻ�����ΪMg3N2��Mg3N2Ϊ���ӻ����Ϊ���Ӿ��壬Mg3N2��ˮ��Ӧ����������þ�Ͱ����������ӷ���ʽΪ��Mg3N2+6H2O=3Mg��OH��2+2NH3����
�ʴ�Ϊ��Mg��Al��Na�����ӣ�Mg3N2+6H2O=3Mg��OH��2+2NH3����
��4����֪N2��g��+3H2��g��?2NH3��g����H=-akJ/mol�������ʽK=
��
���º����£�2molNH3��ȫת������ߣ��ɵ�3molH2��1molN2������ԭƽ��Ϊ��Чƽ�⣬ƽ��ʱNH3��Ũ����ͬ����ƽ��ʱ���������ʵ���Ϊymol���������г���1molN2��3molH2��ƽ��ʱ�μӷ�Ӧ�ĵ���Ϊ��1-y��mol������N2��g��+3H2��g�� 2NH3��g������H=-akJ/mol����֪�ų�������Ϊ��1-y��mol��akJ/mol=a��1-y��kJ����a��1-y��=b��
2NH3��g������H=-akJ/mol����֪�ų�������Ϊ��1-y��mol��akJ/mol=a��1-y��kJ����a��1-y��=b��
�������г���2molNH3������ƽ��ʱ�����ɵ���ymol������N2��g��+3H2��g�� 2NH3��g������H=-akJ/mol����֪���յ�����Ϊymol��akJ/mol=aykJ����c=ay������a=b+c��
2NH3��g������H=-akJ/mol����֪���յ�����Ϊymol��akJ/mol=aykJ����c=ay������a=b+c��
�ʴ�Ϊ��
��a=b+c��
Y�Ļ�̬ԭ�����������������ڲ����������2.5������Yԭ�Ӻ�����2�����Ӳ㣬����������Ϊ5����YΪNԪ�أ�
Z�Ļ�̬ԭ�Ӽ۵����Ų�Ϊnsn-1��ԭ������������Ԫ�أ���n=3��Z�Ļ�̬ԭ�Ӽ۵����Ų�Ϊ3s2����ZΪMgԪ�أ�
W���ʳ��ڻ�ɽ�ڸ��������֣���������������������Ҫԭ��֮һ����WΪSԪ�أ�
��1��YΪNԪ�أ��ڵڶ����ڣ���VA�壻NԪ��ԭ�ӵ�2p�������3�������ӣ���δ�ɶԵ�����Ϊ3��
�ʴ�Ϊ������VA��3��
��2��XΪCԪ�أ�WΪSԪ�أ��ǽ�����S��ǿ����X�ĵ縺�Ա�W��С��Y�������̬�⻯��ΪNH3�����Ӽ����γ������CH4�ķ��Ӽ䲻���γ����������Y�������̬�⻯���X�������̬�⻯����Һ����
�ʴ�Ϊ��С��������֮������γ������
��3��ͬ�����������Ԫ�ص�һ�����ܳ��������ƣ�����IIA����IIIA�壬��VA����VIA�巴�������һ�����ܵĴ�С��ϵΪ��Mg��Al��Na��Y��Z�γɵĻ�����ΪMg3N2��Mg3N2Ϊ���ӻ����Ϊ���Ӿ��壬Mg3N2��ˮ��Ӧ����������þ�Ͱ����������ӷ���ʽΪ��Mg3N2+6H2O=3Mg��OH��2+2NH3����
�ʴ�Ϊ��Mg��Al��Na�����ӣ�Mg3N2+6H2O=3Mg��OH��2+2NH3����
��4����֪N2��g��+3H2��g��?2NH3��g����H=-akJ/mol�������ʽK=
| c2(NH3) |
| c(N2)?c3(H2) |
���º����£�2molNH3��ȫת������ߣ��ɵ�3molH2��1molN2������ԭƽ��Ϊ��Чƽ�⣬ƽ��ʱNH3��Ũ����ͬ����ƽ��ʱ���������ʵ���Ϊymol���������г���1molN2��3molH2��ƽ��ʱ�μӷ�Ӧ�ĵ���Ϊ��1-y��mol������N2��g��+3H2��g��
 2NH3��g������H=-akJ/mol����֪�ų�������Ϊ��1-y��mol��akJ/mol=a��1-y��kJ����a��1-y��=b��
2NH3��g������H=-akJ/mol����֪�ų�������Ϊ��1-y��mol��akJ/mol=a��1-y��kJ����a��1-y��=b���������г���2molNH3������ƽ��ʱ�����ɵ���ymol������N2��g��+3H2��g��
 2NH3��g������H=-akJ/mol����֪���յ�����Ϊymol��akJ/mol=aykJ����c=ay������a=b+c��
2NH3��g������H=-akJ/mol����֪���յ�����Ϊymol��akJ/mol=aykJ����c=ay������a=b+c���ʴ�Ϊ��
| c2(NH3) |
| c(N2)?c3(H2) |
���������⿼��ṹ����λ�ù�ϵӦ�á��縺�ԡ������ܡ�����ʽ����д����ѧƽ��ļ���ȣ��Ѷ��еȣ�ע�⣨4���е�Чƽ��ԭ����Ӧ�ã�

��ϰ��ϵ�д�
�����Ŀ
�������ʲ����ڵȵ�����һ����ǣ�������
| A��CH4��NH4+ |
| B��SO3��CO32- |
| C��CCl4��SO42- |
| D��H2O��CH4 |
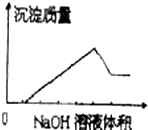 ��ij��ɫ��Һ�л����ص���NaOH��Һֱ����������������������������NaOH��Һ����Ĺ�ϵ��ͼ��ʾ���ɴ�ȷ����ԭ��Һ�к��е������ӿ����ǣ�������
��ij��ɫ��Һ�л����ص���NaOH��Һֱ����������������������������NaOH��Һ����Ĺ�ϵ��ͼ��ʾ���ɴ�ȷ����ԭ��Һ�к��е������ӿ����ǣ�������| A��Mg2+��Al3+��Fe3+ |
| B��Na+��Mg2+��Al3+ |
| C��H+��Ba2+��Al3+ |
| D��H+��Mg2+��Al3+ |
����ʵ������д�����ǣ�������
| A����ȡ��ˮ�еĵⵥ��ʱ��Ӧѡ���л���ȡ��������ȡ����ˮ������ |
| B����������ʱ��Ӧʹ������е�ˮ����ȫ���ɺ���ֹͣ���� |
| C����Һ����ʱ����Һ©�����²�Һ����¿ڷų����ϲ�Һ����Ͽڵ��� |
| D���������ʱ������ˮ����Ӧ���������� |
����ʵ���������ȷ���ǣ�������
| A����������ʱ��Ӧʹ������е�ˮ����ȫ���ɺ���ֹͣ���� |
| B���������ʱ��Ӧʹ�¶ȼ�ˮ����������ƿ��֧�ܿڴ� |
| C����Һ����ʱ���Ƚ���Һ©�����²�Һ����¿�ȫ���ų����ٽ��ϲ�Һ����¿ڷų� |
| D����ȡ����ʱ��Ӧѡ���л���ȡ��������ȡ�����ܶȱ����ˮ�� |
�����Ѿ��ܷⴢ��ʱ��Խ������ζԽŨ������ԭ���Ǿ��õ����Ѿ��У���ԭ����Ⱥ��и���ģ�������
| A���������� | B���Ҵ� |
| C�������� | D������ |
�����뻯ѧ��Ӧ�����仯��ص�������ȷ���ǣ�������
| A��������������һ�����ڷ�Ӧ�������� |
| B��Ӧ�ø�˹���ɣ��ɼ���ijЩ����ֱ�Ӳ����ķ�Ӧ���ʱ� |
| C�����ȷ�Ӧ�ķ�Ӧ�������Ǵ������ȷ�Ӧ�ķ�Ӧ���� |
| D��ͬ��ͬѹ�£�H2��g��+Cl2��g���T2HCl��g���ڹ��պ͵�ȼ�����ġ�Hͬ |